J Vet Sci.
2014 Mar;15(1):157-161. 10.4142/jvs.2014.15.1.157.
Effect of Harderian adenectomy on the statistical analyses of mouse brain imaging using positron emission tomography
- Affiliations
-
- 1Department of Veterinary Medicine, College of Veterinary Medicine, Konkuk University, Seoul 143-701, Korea. ssnahm@konkuk.ac.kr
- 2Molecular Imaging Research Center, Korea Institute of Radiological and Medical Sciences, Seoul 139-706, Korea.
- KMID: 1737622
- DOI: http://doi.org/10.4142/jvs.2014.15.1.157
Abstract
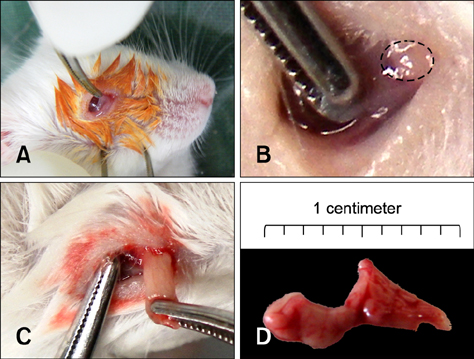
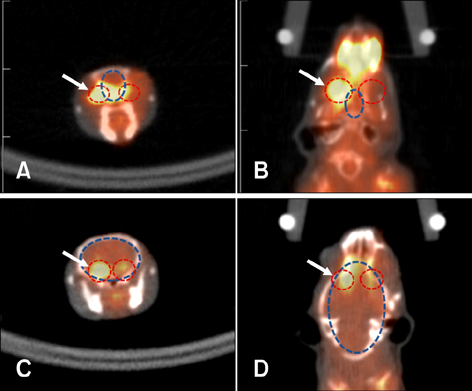
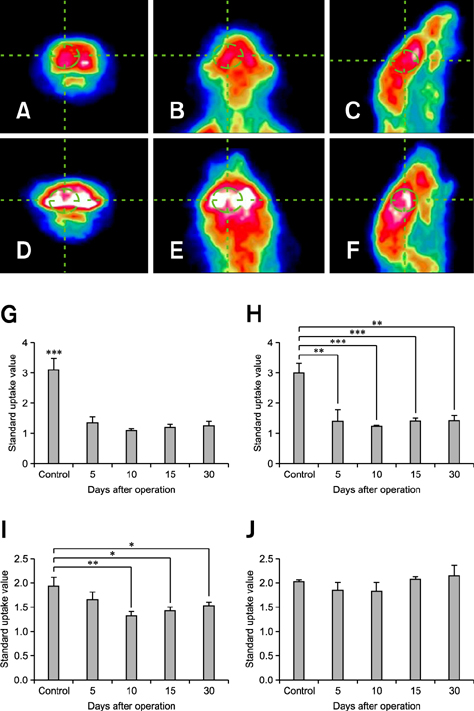
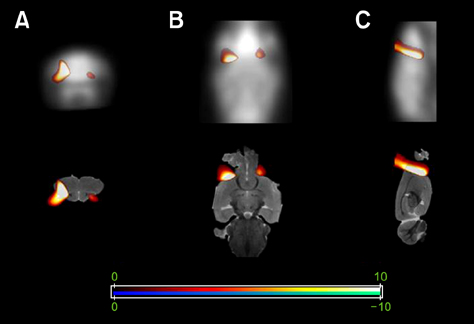
- Positron emission tomography (PET) using 2-deoxy-2-[18F] fluoro-D-glucose (FDG) as a radioactive tracer is a useful technique for in vivo brain imaging. However, the anatomical and physiological features of the Harderian gland limit the use of FDG-PET imaging in the mouse brain. The gland shows strong FDG uptake, which in turn results in distorted PET images of the frontal brain region. The purpose of this study was to determine if a simple surgical procedure to remove the Harderian gland prior to PET imaging of mouse brains could reduce or eliminate FDG uptake. Measurement of FDG uptake in unilaterally adenectomized mice showed that the radioactive signal emitted from the intact Harderian gland distorts frontal brain region images. Spatial parametric measurement analysis demonstrated that the presence of the Harderian gland could prevent accurate assessment of brain PET imaging. Bilateral Harderian adenectomy efficiently eliminated unwanted radioactive signal spillover into the frontal brain region beginning on postoperative Day 10. Harderian adenectomy did not cause any post-operative complications during the experimental period. These findings demonstrate the benefits of performing a Harderian adenectomy prior to PET imaging of mouse brains.
Keyword
MeSH Terms
-
Animals
Brain/*metabolism/radionuclide imaging
Fluorodeoxyglucose F18/*diagnostic use
Frontal Lobe/metabolism/radionuclide imaging
Harderian Gland/metabolism/radionuclide imaging/*surgery
Mice
Mice, Inbred BALB C
Neuroimaging/standards/*veterinary
Positron-Emission Tomography/*veterinary
Radiopharmaceuticals/*diagnostic use
Fluorodeoxyglucose F18
Radiopharmaceuticals
Figure
Reference
-
1. Brammer DW, Riley JM, Kreuser SC, Zasadny KR, Callahan MJ, Davis MD. Harderian gland adenectomy: a method to eliminate confounding radio-opacity in the assessment of rat brain metabolism by 18F-fluoro-2-deoxy-Dglucose positron emission tomography. J Am Assoc Lab Anim Sci. 2007; 46:42–45.2. Deroose CM, De A, Loening AM, Chow PL, Ray P, Chatziioannou AF, Gambhir SS. Multimodality imaging of tumor xenografts and metastases in mice with combined small-animal PET, small-animal CT, and bioluminescence imaging. J Nucl Med. 2007; 48:295–303.3. Fueger BJ, Czernin J, Hildebrandt I, Tran C, Halpern BS, Stout D, Phelps ME, Weber WA. Impact of animal handling on the results of 18F-FDG PET studies in mice. J Nucl Med. 2006; 47:999–1006.4. Fukuyama H, Hayashi T, Katsumi Y, Tsukada H, Shibasaki H. Issues in measuring glucose metabolism of rat brain using PET: the effect of Harderian glands on the frontal lobe. Neurosci Lett. 1998; 255:99–102.
Article5. Kuge Y, Minematsu K, Hasegawa Y, Yamaguchi T, Mori H, Matsuura H, Hashimoto N, Miyake Y. Positron emission tomography for quantitative determination of glucose metabolism in normal and ischemic brains in rats: an insoluble problem by the Harderian glands. J Cereb Blood Flow Metab. 1997; 17:116–120.
Article6. Lancelot S, Zimmer L. Small-animal positron emission tomography as a tool for neuropharmacology. Trends Pharmacol Sci. 2010; 31:411–417.
Article7. Manook A, Yousefi BH, Willuweit A, Platzer S, Reder S, Voss A, Huisman M, Settles M, Neff F, Velden J, Schoor M, von der Kammer H, Wester HJ, Schwaiger M, Henriksen G, Drzezga A. Small-animal PET imaging of amyloid-beta plaques with [11C]PiB and its multi-modal validation in an APP/PS1 mouse model of Alzheimer's disease. PLoS One. 2012; 7:e31310.8. Martic-Kehl MI, Ametamey SM, Alf MF, Schubiger PA, Honer M. Impact of inherent variability and experimental parameters on the reliability of small animal PET data. EJNMMI Res. 2012; 2:26.
Article9. Nikolaus S, Larisch R, Beu M, Hamacher K, Forutan F, Vosberg H, Müller HW. In vivo measurement of D2 receptor density and affinity for 18F-(3-N-methyl)benperidol in the rat striatum with a PET system for small laboratory animals. J Nucl Med. 2003; 44:618–624.10. Payne AP. The harderian gland: a tercentennial review. J Anat. 1994; 185(Pt 1):1–49.11. Pinto LH, Enroth-Cugell C. Tests of the mouse visual system. Mamm Genome. 2000; 11:531–536.
Article12. Soret M, Bacharach SL, Buvat I. Partial-volume effect in PET tumor imaging. J Nucl Med. 2007; 48:932–945.
Article13. Virdee K, Cumming P, Caprioli D, Jupp B, Rominger A, Aigbirhio FI, Fryer TD, Riss PJ, Dalley JW. Applications of positron emission tomography in animal models of neurological and neuropsychiatric disorders. Neurosci Biobehav Rev. 2012; 36:1188–1216.
Article14. Zovein A, Flowers-Ziegler J, Thamotharan S, Shin D, Sankar R, Nguyen K, Gambhir S, Devaskar SU. Postnatal hypoxic-ischemic brain injury alters mechanisms mediating neuronal glucose transport. Am J Physiol Regul Integr Comp Physiol. 2004; 286:R273–R282.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Methodological Review on Functional Neuroimaging Using Positron Emission Tomography
- A Case of Neuro-Behcet's Disease: Comparison of Neurological Symptoms with PET, SPECT, and MRI Findings
- A Review of Machine Learning Approaches for Brain Positron Emission Tomography Data Analysis
- Nuclear Neurology
- Perspectives in TSPO PET Imaging for Neurologic Diseases