J Korean Med Sci.
2004 Oct;19(5):724-728. 10.3346/jkms.2004.19.5.724.
Increased Releasability of Skin Mast Cells after Exercise in Patients with Exercise-induced Asthma
- Affiliations
-
- 1Department of Allergy, Chonnam National University Medical School, Research Institute of Medical Science, Gwangju, Korea. ischoi@chonnam.chonnam.ac.kr
- KMID: 1733516
- DOI: http://doi.org/10.3346/jkms.2004.19.5.724
Abstract
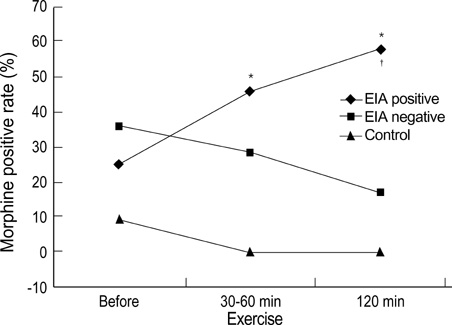
- The role of lung mast cells in exercise-induced asthma (EIA) is controversial. To investigate whether the skin mast cell releasability is increased after exercise in EIA, 49 young atopic men with or without asthma took part in a free-running test for 6 min and were given skin prick tests using morphine, a mast cell secretagogue, before and after the exercise. The mean diameters of the wheal induced by morphine in patients with EIA were not significantly different from those in patients without EIA before exercise, although the baseline lung function was significantly lower and the airway hyperresponsiveness, the peripheral blood eosinophil count, and the size of the wheal in response to Dermatophagoides pteronyssinus were significantly higher in patients with EIA. However, the differences of the morphine-induced wheal diameter between patients with EIA and those without EIA became significant at 120 min after exercise (p<0.05), while the responses to histamine were not significantly different. These results suggest that exercise increases the releasability of skin mast cells in EIA patients whose asthma/allergy are relatively severe.
Keyword
MeSH Terms
Figure
Reference
-
1. Anderson SD. Is there a unifying hypothesis for exercise-induced asthma? J Allergy Clin Immunol. 1984. 73:660–665.
Article2. Sheppard D, Eschenbacher WL. Respiratory water loss as a stimulus to exercise-induced bronchoconstriction. J Allergy Clin Immunol. 1984. 73:640–642.
Article3. Lee TH, Brown MJ, Nagy L, Causon R, Walport MJ, Kay AB. Exercise-induced release of histamine and neutrophil chemotactic factor in atopic asthmatics. J Allergy Clin Immunol. 1982. 70:73–81.
Article4. O'Sullivan S, Roquet A, Dahlen B, Larsen F, Eklund A, Kumlin M, O'Byrne PM, Dahlen SE. Evidence for mast cell activation during exercise-induced bronchoconstriction. Eur Respir J. 1998. 12:345–350.5. Broide DH, Eisman S, Ramsdell JW, Ferguson P, Schwartz LB, Wasserman SI. Airway levels of mast cell-derived mediators in exercise-induced asthma. Am Rev Respir Dis. 1990. 141:563–568.
Article6. Turner CR, Darowski MJ, Sampson HA, Spannhake EW, Hirshman CA. Dermal mast cell releasability and end organ responsiveness in atopic and nonatopic dogs. J Allergy Clin Immunol. 1989. 83:643–648.
Article7. Choi IS, Park SC, Kang KW. Clinical usefulness of morphine skin prick test in diagnosis of allergic diseases. J Asthma Allergy Clin Immunol. 1999. 19:476–483.8. Casale TB, Keahey TM, Kaliner M. Exercise-induced anaphylactic syndromes. Insights into diagnostic and pathophysiologic features. JAMA. 1986. 255:2049–2053.
Article9. Kivity S, Sneh E, Greif J, Topilsky M, Mekori YA. The effect of food and exercise on the skin response to compound 48/80 in patients with food-associated exercise-induced urticaria-angioedema. J Allergy Clin Immunol. 1988. 81:1155–1158.
Article10. National Institutes of Health. NIH NHLBI Revised 2002. NIH Publication No. 02-3659. Global strategy for asthma management and prevention. 2002.11. American Thoracic Society. Standardization of spirometry: 1994 update. Am J Respir Crit Care Med. 1995. 152:1107–1136.12. Sterk PJ, Fabbri LM, Quanjer PH, Cockcroft DW, O'Byrne PM, Anderson SD, Juniper EF, Malo JL. Airway responsiveness: standardized challenge testing with pharmacological, physical and sensitizing stimuli in adults. Eur Respir J Suppl. 1993. 16:Suppl. 53–83.13. Koh YI, Choi IS, Lim H. Atopy may be related to exercise-induced bronchospasm in asthma. Clin Exp Allergy. 2002. 32:532–536.
Article14. Casale TB, Bowman S, Kaliner M. Induction of human cutaneous mast cell degranulation by opiates and endogenous opioid peptides: evidence for opiate and nonopiate receptor participation. J Allergy Clin Immunol. 1984. 73:775–781.
Article15. O'Byrne PM. Middleton E, Reed CE, Ellis EF, Adkinson NF, Yunginger JW, Busse WW, editors. Airway hyperresponsiveness. Allergy principles & practice. 1998. 5th ed. Toronto: Mosby;859–866.16. Godfrey S. McFadden ER, editor. Clincal and physiological features. Exercise-Induced Asthma. 1999. New York: Marcel Dekker, Inc.;11–45.17. Koh YI, Choi IS. Blood eosinophil counts for the prediction of the severity of exercise-induced bronchospasm in asthma. Respir Med. 2002. 96:120–125.
Article18. Kiistala R, Kiistala U. Local cholinergic urticaria at methacholine test site. Acta Derm Venereol. 1997. 77:84–85.19. Lin RY, Barnard M. Skin testing with food, codeine, and histamine in exercise-induced anaphylaxis. Ann Allergy. 1993. 70:475–478.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Late Local Urticaria as a Long-term Sequela of Allergen-Specific Immunotherapy
- Mugwort pollen- induced bnsophil histamine releasability in seasonal allergic rhinitis and asthma
- Exercise induced delayed bronchoconstriction in children with asthma
- Change of Pulmonary Function after Exercise Loading Test and Preventive Measures on Exercise-induced Asthma in Chlidren
- Urinary N-methylhistamine and sulfidopeptide leukotriene in exercise-induced asthma