Korean J Gastroenterol.
2014 Mar;63(3):146-150. 10.4166/kjg.2014.63.3.146.
New Therapeutic Strategies against Helicobacter pylori
- Affiliations
-
- 1Department of Internal Medicine, College of Medicine, The Catholic University of Korea, Seoul, Korea. gastro@catholic.ac.kr
- KMID: 1730925
- DOI: http://doi.org/10.4166/kjg.2014.63.3.146
Abstract
- The standard therapy for Helicobacter pylori infection in Korea is a triple-drug regimen consisting of a proton pump inhibitor with two antibiotics such as clarithromycin, amoxicillin, and metronidazole. However, as the eradication rate of this regimen has declined over the past decade, this prompted the formulation of new therapeutic regimens. New therapeutic strategies against H. pylori infection that had been tried all over the world include sequential therapy, concomitant therapy, and tailored therapy This article will review the basic concepts and the results of previous clinical trials on the aforementioned new therapeutic regiments.
Keyword
MeSH Terms
-
Amoxicillin/pharmacology/therapeutic use
Anti-Bacterial Agents/pharmacology/*therapeutic use
Clarithromycin/pharmacology/therapeutic use
Disease Eradication/trends
Drug Therapy, Combination
Helicobacter Infections/*drug therapy
*Helicobacter pylori/drug effects
Humans
Nitroimidazoles/pharmacology/therapeutic use
Proton Pump Inhibitors/pharmacology/therapeutic use
Amoxicillin
Anti-Bacterial Agents
Clarithromycin
Nitroimidazoles
Proton Pump Inhibitors
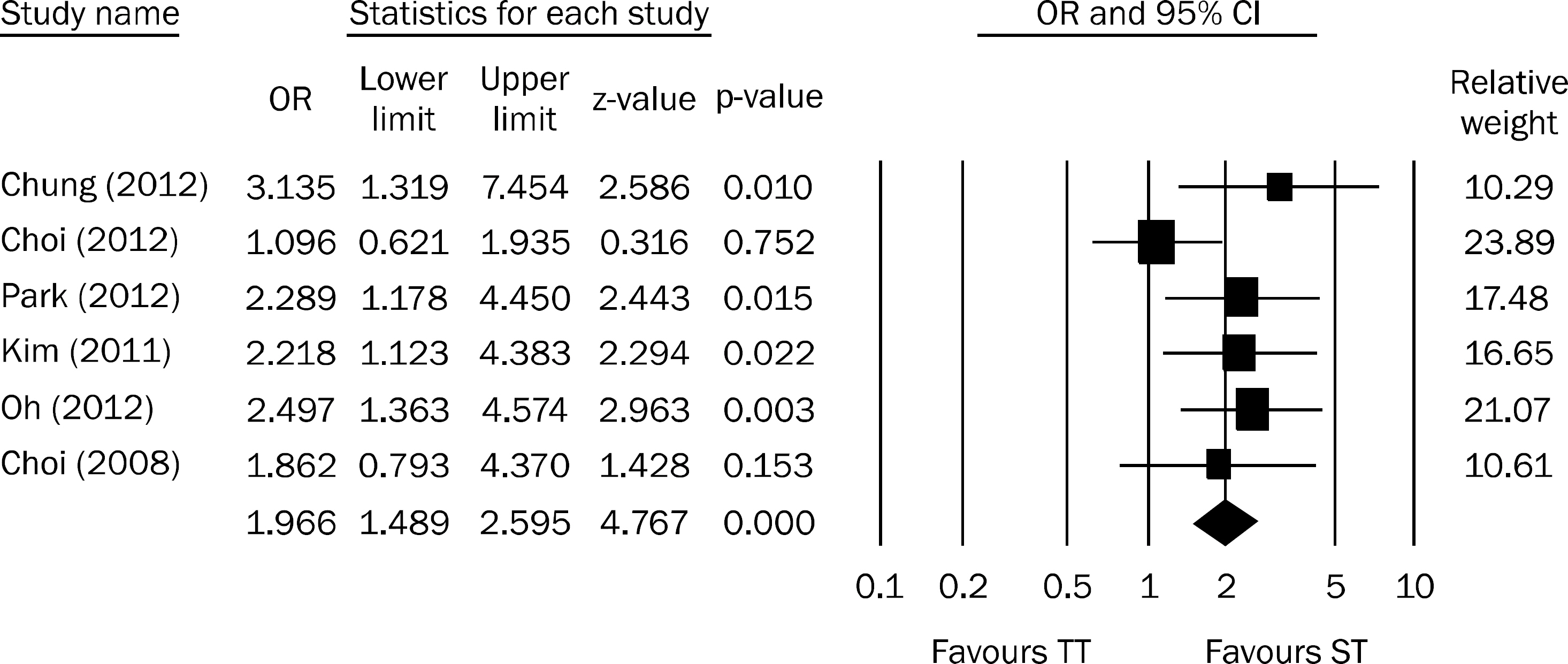
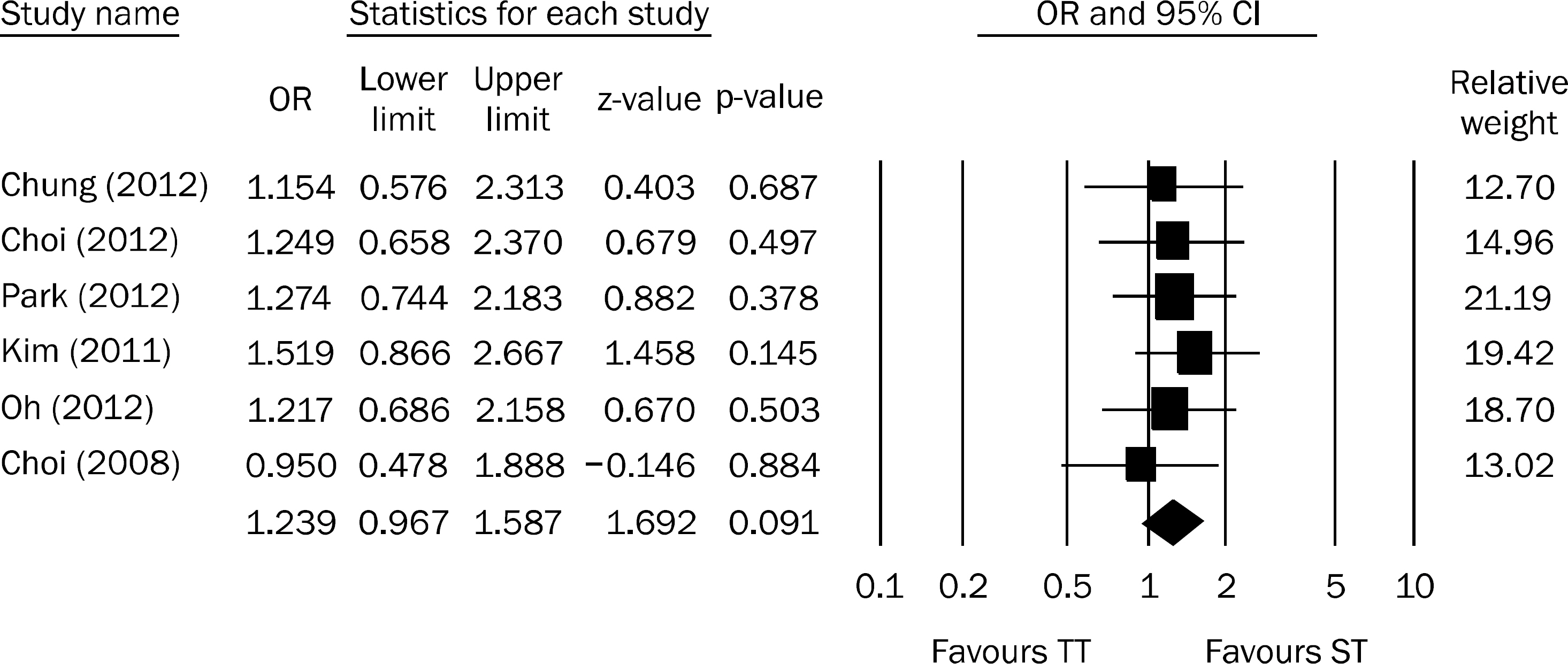
Figure
Reference
-
References
1. Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984; 1:1311–1315.
Article2. Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002; 347:1175–1186.3. Blaser MJ. Helicobacter pylori and gastric diseases. BMJ. 1998; 316:1507–1510.4. Leodolter A, Kulig M, Brasch H, Meyer-Sabellek W, Willich SN, Malfertheiner P. A metaanalysis comparing eradication, healing and relapse rates in patients with Helicobacter pylori-associated gastric or duodenal ulcer. Aliment Pharmacol Ther. 2001; 15:1949–1958.5. Wotherspoon AC, Doglioni C, Diss TC, et al. Regression of primary low-grade B-cell gastric lymphoma of mucosa-associated lymphoid tissue type after eradication of Helicobacter pylori. Lancet. 1993; 342:575–577.6. Hong SS, Jung HY, Choi KD, et al. A prospective analysis of low-grade gastric malt lymphoma after Helicobacter pylori eradication. Helicobacter. 2006; 11:569–573.7. Uemura N, Mukai T, Okamoto S, et al. Effect of Helicobacter pylori eradication on subsequent development of cancer after endoscopic resection of early gastric cancer. Cancer Epidemiol Biomarkers Prev. 1997; 6:639–642.8. Fukase K, Kato M, Kikuchi S, et al. Japan Gast Study Group. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. Lancet. 2008; 372:392–397.9. Korean H. pylori Study Group. Diagnosis and treatment of Helicobacter pylori infection in Korea. Korean J Gastroenterol. 1998; 32:275–289.10. Kim N, Kim JJ, Choe YH, Kim HS, Kim JI, Chung IS. Korean College of Helicobacter and Upper Gastrointestinal Research; Korean Association of Gastroenterology. Diagnosis and treatment guidelines for Helicobacter pylori infection in Korea. Korean J Gastroenterol. 2009; 54:269–278.11. Kim SG, Jung HK, Lee HL, et al. Korean College of Helicobacter and Upper Gastrointestinal Research. Guidelines for the diagnosis and treatment of Helicobacter pylori infection in Korea, 2013 revised edition. Korean J Gastroenterol. 2013; 62:3–26.12. Graham DY, Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut. 2010; 59:1143–1153.13. Chung JW, Lee GH, Han JH, et al. The trends of one-week first-line and second-line eradication therapy for Helicobacter pylori infection in Korea. Hepatogastroenterology. 2011; 58:246–250.14. Chung WC, Lee KM, Paik CN, et al. Inter-departmental differences in the eradication therapy for Helicobacter pylori infection: a single center study. Korean J Gastroenterol. 2009; 53:221–227.15. Choi YS, Cheon JH, Lee JY, et al. The trend of eradication rates of first-line triple therapy for Helicobacter pylori infection: single center experience for recent eight years. Korean J Gastroenterol. 2006; 48:156–161.16. Zullo A, Rinaldi V, Winn S, et al. A new highly effective short-term therapy schedule for Helicobacter pylori eradication. Aliment Pharmacol Ther. 2000; 14:715–718.17. Zullo A, De Francesco V, Hassan C, Morini S, Vaira D. The sequential therapy regimen for Helicobacter pylori eradication: a pooled-data analysis. Gut. 2007; 56:1353–1357.
Article18. Mégraud F. H pylori antibiotic resistance: prevalence, importance, and advances in testing. Gut. 2004; 53:1374–1384.19. Perri F, Clemente R, Festa V, et al. Relationship between the results of pretreatment urea breath test and efficacy of eradication of Helicobacter pylori infection. Ital J Gastroenterol Hepatol. 1998; 30:146–150.20. Maconi G, Parente F, Russo A, Vago L, Imbesi V, Bianchi Porro G. Do some patients with Helicobacter pylori infection benefit from an extension to 2 weeks of a proton pump inhibitor-based triple eradication therapy? Am J Gastroenterol. 2001; 96:359–366.21. Lai YC, Yang JC, Huang SH. Pretreatment urea breath test results predict the efficacy of Helicobacter pylori eradication therapy in patients with active duodenal ulcers. World J Gastroenterol. 2004; 10:991–994.22. Paulsen IT, Brown MH, Skurray RA. Proton-dependent multidrug efflux systems. Microbiol Rev. 1996; 60:575–608.
Article23. Webber MA, Piddock LJ. The importance of efflux pumps in bacterial antibiotic resistance. J Antimicrob Chemother. 2003; 51:9–11.
Article24. Roberts MC. Resistance to macrolide, lincosamide, streptogra-min, ketolide, and oxazolidinone antibiotics. Mol Biotechnol. 2004; 28:47–62.
Article25. Murakami K, Fujioka T, Okimoto T, Sato R, Kodama M, Nasu M. Drug combinations with amoxycillin reduce selection of clarithromycin resistance during Helicobacter pylori eradication therapy. Int J Antimicrob Agents. 2002; 19:67–70.26. De Francesco V, Margiotta M, Zullo A, et al. Clarithromycin-resistant genotypes and eradication of Helicobacter pylori. Ann Intern Med. 2006; 144:94–100.27. Kim JS, Kim BW, Ham JH, et al. Sequential therapy for Helicobacter pylori infection in Korea: systematic review and metaanalysis. Gut Liver. 2013; 7:546–551.28. Kim JM, Kim JS, Jung HC, Kim N, Kim YJ, Song IS. Distribution of antibiotic MICs for Helicobacter pylori strains over a 16-year period in patients from Seoul, South Korea. Antimicrob Agents Chemother. 2004; 48:4843–4847.29. Hwang TJ, Kim N, Kim HB, et al. Change in antibiotic resistance of Helicobacter pylori strains and the effect of A2143G point mutation of 23S rRNA on the eradication of H. pylori in a single center of Korea. J Clin Gastroenterol. 2010; 44:536–543.30. Okada M, Oki K, Shirotani T, et al. A new quadruple therapy for the eradication of Helicobacter pylori. Effect of pretreatment with omeprazole on the cure rate. J Gastroenterol. 1998; 33:640–645.31. Treiber G, Ammon S, Schneider E, Klotz U. Amoxicillin/metronidazole/omeprazole/clarithromycin: a new, short quadruple therapy for Helicobacter pylori eradication. Helicobacter. 1998; 3:54–58.32. Kim SY, Lee SW, Hyun JJ, et al. Comparative study of Helicobacter pylori eradication rates with 5-day quadruple "concomitant" therapy and 7-day standard triple therapy. J Clin Gastroenterol. 2013; 47:21–24.33. Essa AS, Kramer JR, Graham DY, Treiber G. Meta-analysis: four-drug, three-antibiotic, non-bismuth-containing "concomitant therapy" versus triple therapy for Helicobacter pylori eradication. Helicobacter. 2009; 14:109–118.34. Gisbert JP, Calvet X. Review article: non-bismuth quadruple (concomitant) therapy for eradication of Helicobater pylori. Aliment Pharmacol Ther. 2011; 34:604–617.35. Lim JH, Lee DH, Choi C, et al. Clinical outcomes of two-week sequential and concomitant therapies for Helicobacter pylori eradication: a randomized pilot study. Helicobacter. 2013; 18:180–186.36. McNicholl AG, Marin AC, Molina-Infante J, et al. Participant Centres. Randomised clinical trial comparing sequential and concomitant therapies for Helicobacter pylori eradication in routine clinical practice. Gut. 2014; 63:244–249.37. Huang YK, Wu MC, Wang SS, et al. Lansoprazole-based sequential and concomitant therapy for the first-line Helicobacter pylori eradication. J Dig Dis. 2012; 13:232–238.38. Wu DC, Hsu PI, Wu JY, et al. Sequential and concomitant therapy with four drugs is equally effective for eradication of H pylori infection. Clin Gastroenterol Hepatol. 2010; 8:36–41.39. Woo HY, Park DI, Park H, et al. Dual-priming oligonucleotide-based multiplex PCR for the detection of Helicobacter pylori and determination of clarithromycin resistance with gastric biopsy specimens. Helicobacter. 2009; 14:22–28.40. Lee HJ, Kim JI, Cheung DY, et al. Eradication of Helicobacter pylori according to 23S ribosomal RNA point mutations associated with clarithromycin resistance. J Infect Dis. 2013; 208:1123–1130.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immune Response to Helicobacter pylori Infection
- Perspective of Helicobacter pylori Research: Molecular Pathogenesis of Helicobacter pylori Virulence Factors
- Response to Treatment of Helicobacter pylori-associated Dyspepsia: Eradication of Helicobacter pylori or Correction of Gastric or Intestinal Dysbiosis?
- Treatment of Helicobacter pylori infection in functional dyspepsia
- Helicobacter pylori Infection and Cardiovascular Disease