J Korean Med Sci.
2010 May;25(5):766-771. 10.3346/jkms.2010.25.5.766.
Changes of Antimicrobial Peptides and Transepidermal Water Loss After Topical Application of Tacrolimus and Ceramide-dominant Emollient in Patients with Atopic Dermatitis
- Affiliations
-
- 1Department of Dermatology, Chung-Ang University College of Medicine, Seoul, Korea. drseo@hanafos.com
- 2Chung-Ang Medical Research Center, Chung-Ang University College of Medicine, Seoul, Korea.
- KMID: 1713963
- DOI: http://doi.org/10.3346/jkms.2010.25.5.766
Abstract
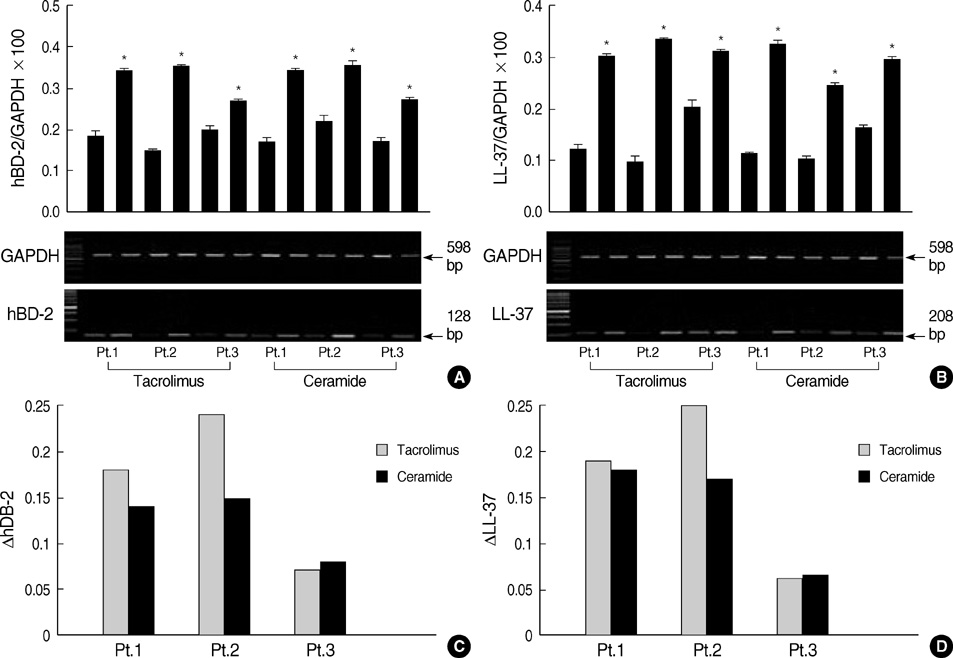
- Increased transepidermal water loss (TEWL) and downregulated antimicrobial peptides (AMPs) are observed in patients with atopic dermatitis (AD). Tacrolimus and ceramide-dominant emollients are effective in the treatment of AD by preventing the production of inflammatory cytokines and by correcting skin barrier dysfunctions, respectively. Present study was designed to investigate the relationship between antimicrobial and barrier factors by measuring the changes of AMPs and TEWL after topical application of tacrolimus and ceramide-dominant emollient in the patients with AD. A total of three patients with AD were treated with tacrolimus in one lesion and ceramide-dominant emollient in another lesion for 4 weeks. RT-PCR and western blotting revealed that the mRNA and protein expression levels of hBD-2 and LL-37 were increased on the both study sites. Immunohistochemical analysis showed significant increase of AMPs and IL-1alpha, while, IL-4 was decreased on the both study sites. The mean changes of TEWL and AMPs showed no statistical difference between both sites. Tacrolimus and ceramide-dominant emollient influence on both TEWL and AMPs expression in patients with AD, namely they have similar effects on both of the two. This study shows that restoration of permeability barrier function is accompanied by the concomitant improvement of antimicrobial defense in patients with AD.
MeSH Terms
-
Administration, Topical
Adolescent
Antimicrobial Cationic Peptides/*metabolism
Ceramides/*administration & dosage
Dermatitis, Atopic/*drug therapy/*metabolism
Emollients/administration & dosage
Female
Humans
Immunosuppressive Agents/administration & dosage
Male
Skin Absorption/*drug effects
Tacrolimus/*administration & dosage
Treatment Outcome
Water Loss, Insensible/*drug effects
Young Adult
Antimicrobial Cationic Peptides
Ceramides
Emollients
Immunosuppressive Agents
Tacrolimus
Figure
Cited by 1 articles
-
Clinical use of a ceramide-based moisturizer for treating dogs with atopic dermatitis
Ji-young Jung, Eui-hwa Nam, Seol-hee Park, Seung-hee Han, Cheol-yong Hwang
J Vet Sci. 2013;14(2):199-205. doi: 10.4142/jvs.2013.14.2.199.
Reference
-
1. Leung DY. Atopic dermatitis: new insights and opportunities for therapeutic intervention. J Allergy Clin Immunol. 2000. 105:860–876.
Article2. Williams HC. Is the prevalence of atopic dermatitis increasing? Clin Exp Dermatol. 1992. 17:385–391.
Article3. Elias PM, Wood LC, Feingold KR. Epidermal pathogenesis of inflammatory dermatoses. Am J Contact Dermat. 1999. 10:119–126.
Article4. Elias PM, Steinhoff M. "Outside-to-inside" (and now back to "outside") pathogenic mechanisms in atopic dermatitis. J Invest Dermatol. 2008. 128:1067–1070.
Article5. Kurahashi R, Hatano Y, Katagiri K. IL-4 suppresses the recovery of cutaneous permeability barrier functions in vivo. J Invest Dermatol. 2008. 128:1329–1331.
Article6. Hatano Y, Terashi H, Arakawa S, Katagiri K. Interleukin-4 suppresses the enhancement of ceramide synthesis and cutaneous permeability barrier functions induced by tumor necrosis factor-alpha and interferon-gamma in human epidermis. J Invest Dermatol. 2005. 124:786–792.7. Elias PM. Stratum corneum defensive functions: an integrated view. J Invest Dermatol. 2005. 125:183–200.
Article8. Oren A, Ganz T, Liu L, Meerloo T. In human epidermis, beta-defensin 2 is packaged in lamellar bodies. Exp Mol Pathol. 2003. 74:180–182.9. Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, Gallo RL, Leung DY. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002. 347:1151–1160.
Article10. Aberg KM, Man MQ, Gallo RL, Ganz T, Crumrine D, Brown BE, Choi EH, Kim DK, Schröder JM, Feingold KR, Elias PM. Co-regulation and interdependence of the mammalian epidermal permeability and antimicrobial barriers. J Invest Dermatol. 2008. 128:917–925.
Article11. Elias PM, Choi EH. Interactions among stratum corneum defensive functions. Exp Dermatol. 2005. 14:719–726.
Article12. Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol Suppl (Stockh). 1980. 92:44–47.13. Pinnagoda J, Tupker RA, Agner T, Serup J. Guidelines for transepidermal water loss (TEWL) measurement. A report from the Standardization Group of the European Society of Contact Dermatitis. Contact Dermatitis. 1990. 22:164–178.14. Howell MD, Jones JF, Kisich KO, Streib JE, Gallo RL, Leung DY. Selective killing of vaccine virus by LL-37: implications for eczema vaccinatum. J Immunol. 2004. 172:1763–1767.15. Kim JE, Kim BJ, Jeong MS, Seo SJ, Kim MN, Hong CK, Ro BI. Expression and modulation of LL-37 in normal human keratinocytes, HaCaT cells, and inflammatory skin diseases. J Korean Med Sci. 2005. 20:649–654.
Article16. Rieg S, Steffen H, Seeber S, Humeny A, Kalbacher H, Dietz K, Garbe C, Schittek B. Deficiency of dermcidin-derived antimicrobial peptides in sweat of patients with atopic dermatitis correlates with an impaired innate defense of human skin in vivo. J Immunol. 2005. 174:8003–8010.
Article17. Boguniewicz M. Update on atopic dermatitis: insights into pathogenesis and new treatment paradigms. Allergy Asthma Proc. 2004. 25:279–282.18. Raychaudhuri SP, Jiang WY, Raychaudhuri SK, Krensky AM. Lesional T cells and dermal dendrocytes in psoriasis plaque express increased levels of granulysin. J Am Acad Dermatol. 2004. 51:1006–1008.
Article19. Fellermann K, Wehkamp J, Stange EF. Antimicrobial peptides in the skin. N Engl J Med. 2003. 348:361–363.
Article20. Nomura I, Goleva E, Howell MD, Hamid QA, Ong PY, Hall CF, Darst MA, Gao B, Boguniewicz M, Travers JB, Leung DY. Cytokine milieu of atopic dermatitis, as compared to psoriasis, skin prevents induction of innate immune response genes. J Immunol. 2003. 171:3262–3269.
Article21. Loden M, Andersson AC, Lindberg M. Improvement in skin barrier function in patients with atopic dermatitis after treatment with a moisturizing cream (Canoderm). Br J Dermatol. 1999. 140:264–267.22. Chamlin SL, Frieden IJ, Fowler A, Williams M, Kao J, Sheu M, Elias PM. Ceramide-dominant, barrier-repair lipids improve childhood atopic dermatitis. Arch Dermatol. 2001. 137:1110–1112.23. Nghiem P, Pearson G, Langley RG. Tacrolimus and pimecrolimus: from clever prokaryotes to inhibiting calcineurin and treating atopic dermatitis. J Am Acad Dermatol. 2002. 46:228–241.
Article24. Kis K, Bodai L, Polyanka H, Eder K, Pivarcsi A, Duda E, Soos G, Bata-Csorgo Z, Kemeny L. Budesonide, but not tacrolimus, affects the immune functions of normal human keratinocytes. Int Immunopharmacol. 2006. 6:358–368.
Article25. Reilly DM, Parslew R, Sharpe GR, Powell S, Green MR. Inflammatory mediators in normal, sensitive and diseased skin types. Acta Derm Venereol. 2000. 80:171–174.26. Feingold KR. The regulation and role of epidermal lipid synthesis. Adv Lipid Res. 1991. 24:57–82.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Four Cases of Atopic Dermatitis with Topical Tacrolimus Therapy
- Expression of Antimicrobial Peptides according to Changes of Transepidermal Water Loss Levels in Patients with Atopic Dermatits
- Skin Barrier Dysfunction in the Scalp, Nails, and Lips in Patients with Atopic Dermatitis
- Evaluation of Clinical Manifestation according to 'Dermatitis Mapping' in Atopic Dermatitis
- Effects of Topical N-Acetylcysteine on Skin Hydration/Transepidermal Water Loss in Healthy Volunteers and Atopic Dermatitis Patients