J Korean Med Sci.
2007 Feb;22(1):48-56. 10.3346/jkms.2007.22.1.48.
Effects of Polyamines on Contractility of Guinea-Pig Gastric Smooth Muscle
- Affiliations
-
- 1Department of Physiology, Chungbuk National University, College of Medicine, Cheongju, Korea. physiokyc@chungbuk.ac.kr
- 2Department of Physiology & Biophysics, Seoul National University College of Medicine, Seoul, Korea.
- 3Department of Otolaryngology, Seoul Municipal Boramae Hospital, Seoul National University College of Medicine, Seoul, Korea.
- 4Department of Physiology, College of Medicine, Kwandong University, Gangneung, Korea.
- 5Department of Biochemistry, Chungbuk National University, College of Medicine, Cheongju, Korea.
- 6Department of Internal Medicine, Chungbuk National University, College of Medicine, Cheongju, Korea.
- 7Department of Surgery, Chungbuk National University, College of Medicine, Cheongju, Korea.
- 8Department of Physiology, Medical School, Shanghai Jiaotong University, Shanghai, China.
- KMID: 1713229
- DOI: http://doi.org/10.3346/jkms.2007.22.1.48
Abstract
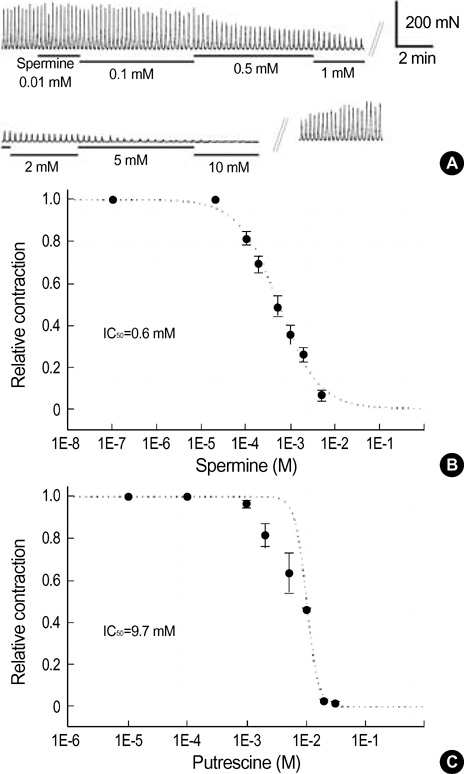
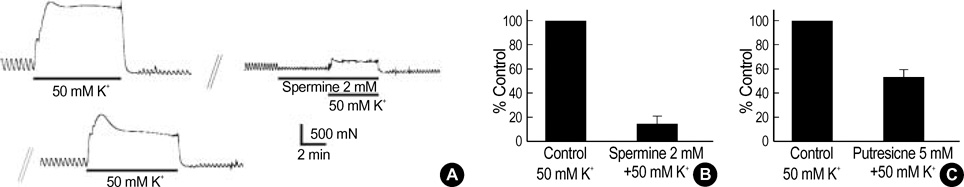
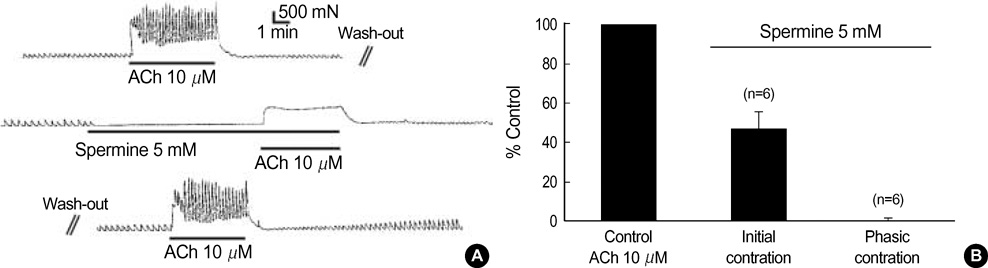
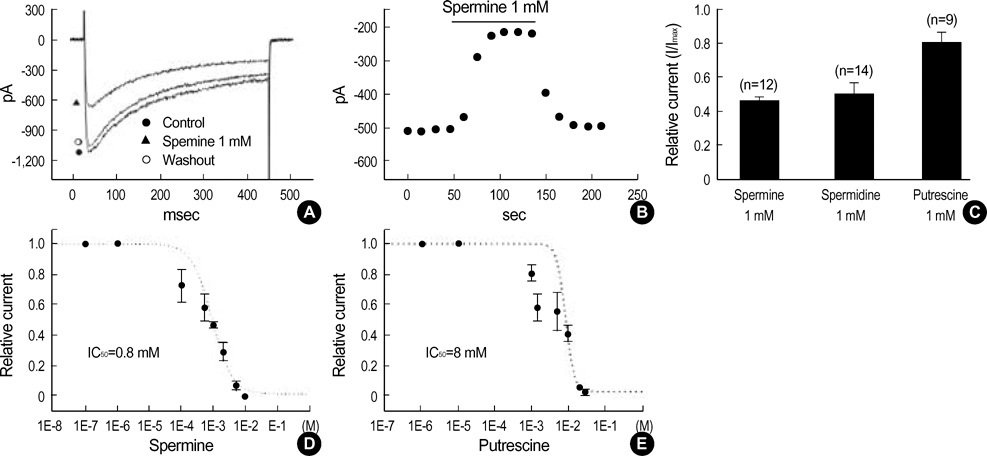
- This study was designed to investigate the effects of polyamines on mechanical contraction and voltage-dependent calcium current (VDCC) of guinea-pig gastric smooth muscle. Mechanical contraction and calcium channel current (I(Ba)) were recorded by isometric tension recording and whole-cell patch clamp technique. Spermine, spermidine and putrescine inhibited spontaneous contraction of the gastric smooth muscle in a concentration-dependent manner. Spermine (2 mM) reduced high K+ (50 mM)-induced contraction to 16+/-6.4% of the control (n=9), and significantly inhibited I(Ba) in a reversible manner (p<0.05; IC50=0.8 mM). Pre- and post-treatment of tissue with spermine (2-5 mM, n=10) also inhibited acetylcholine (10 micrometer)-induced phasic contraction to 5+/-6.4% of the control. Inhibitory effect of spermine on I(Ba) was observed at a wide range of test potentials of current/voltage (I/V) relationship (p<0.05), and steady-state activation of I(Ba) was shifted to the right by spermine (p<0.05). Spermidine and putrescine (1 mM each) also inhibited I(Ba) to 51+/-5.7% and 81+/-5.3% of the control, respectively. And putrescine (1 mM) inhibited I(Ba) at whole tested potentials (p<0.05) without significant change of kinetics (p<0.05). Finally, 5 mM putrescine also inhibited high K+ -induced contraction to 53+/-7.1% of the control (n=4). These findings suggest that polyamines inhibit contractions of guinea-pig gastric smooth muscle via inhibition of VDCC.
Keyword
MeSH Terms
Figure
Reference
-
1. Igarashi K, Kashiwagi K. Polyamines: mysterious modulators of cellular functions. Biochem Biophys Res Commun. 2000. 271:559–564.
Article2. Nishimura K, Shiina R, Kashiwagi K, Igarashi K. Decrease in polyamines with aging and their ingestion from food and drink. J Biochem. 2006. 139:81–90.
Article3. Wery I, Kaouass M, Deloyer O, Buts JP, Barbason H, Dandrifosse G. Exogenous spermine induces maturation of the liver in suckling rats. Hepatology. 1996. 24:1206–1210.
Article4. Belting M, Persson S, Fransson LA. Proteoglycan involvement in polyamine uptake. Biochem J. 1999. 338:317–323.
Article5. Tabor CW, Tabor H. Polyamines. Annu Rev Biochem. 1984. 53:749–790.
Article6. Nilsson BO, Gomez M, Swärd K, Hellstrand P. Regulation of Ca2+ channel and phosphatase activities by polyamines in intestinal and vascular smooth muscle-implications for cellular growth and contractility. Acta Physiol Scand. 2002. 176:33–41.7. Swärd K, Nilsson BO, Hellstrand P. Polyamines increase Ca2+ sensitivity in permeabilized smooth muscle of guinea-pig ileum. Am J Physiol. 1994. 266:C1754–C1763.8. Tsvilovskyy VV, Zholos AV, Bolton TB. Effects of polyamines on the muscarinic receptor-operated cation current in guinea-pig ileal smooth muscle myocytes. Br J Pharmacol. 2004. 143:968–975.
Article9. Chideckel EW, Fedan JS, Mike P. Polyamines and putrescine relax respiratory tract smooth muscle in the guinea-pig. Eur J Pharmacol. 1985. 116:187–190.10. Hashimoto H, Unemoto T, Hayashi M. Inhibitory action of spermine on the contractions of rat uterus. Am J Physiol. 1973. 225:743–746.
Article11. Maruta K, Mizoguchi Y, Osa T. Effects of polyamines on the mechanical and electrical activities of the isolated circular muscle of rat uterus. Jpn J Physiol. 1985. 36:903–915.
Article12. Nilsson BO, Hellstrand P. Effects of polyamines on intracellular calcium and mechanical activity in smooth muscle of guinea-pig taenia coli. Acta Physiol Scand. 1993. 148:37–43.
Article13. Chideckel EW, Dedhia HH, Fedan JS, Teba L, Jain A. Spermine decreases peripheral vascular resistance in the dog and relaxes the isolated aorta of the guinea pig. Cardiovasc Res. 1986. 20:931–934.
Article14. Gomez M, Hellstrand P. Effects of polyamines on voltage-activated calcium channels in guinea-pig intestinal smooth muscle. Pflugers Arch. 1995. 430:501–507.
Article15. Tansy MF, Martin JS, Landin WE, Kendall FM, Melamed S. Spermine and spermidine as inhibitors of gastrointestinal motor activity. Surg Gynecol Obstet. 1982. 154:74–80.16. Aihara H, Otomo S, Isobe Y, Ohzeki M, Igarashi K, Hirose S. Polyamine inhibition of gastric ulceration and seretion in rats. Biochem Pharmacol. 1983. 32:1733–1736.17. Martin JS, Tansy MF. Comparison of the inhibitory effects of spermine, papaverine and adrenalin upon isolated segments of the rat small intestine. Clin Exp Pharmacol Physiol. 1986. 13:87–90.18. Peulen O, Deloyer P, Dandrifosse G. Short-term effects of spermine ingestion on the small intestine: a comparison of suckling and weaned rats. Reprod Nutr Dev. 2004. 44:353–364.
Article19. Fernandez AI, Cantabrana B, Sanchez M, Hidalgo A. Extracellular and intracellular effects of polyamines on smooth muscle contractions. Life Sci. 1995. 57:855–861.20. Gomez M, Hellstrand P. Endogenous polyamines modulate Ca2+ channel activity in guinea-pig intestinal smooth muscle. Pflugers Arch. 1999. 438:445–451.21. Katzka DA, Morad M. Properties of calcium channels in guinea-pig gastric myocytes. J Physiol. 1989. 413:175–197.
Article22. Kim SJ, Ahn SC, Kim JK, Kim YC, So I, Kim KW. Changes in intracellular Ca2+ concentration induced by L-type Ca2+ channel current in guinea-pig gastric myocytes. Am J Physiol Cell Physiol . 1997. 8273:1947–1956.23. Huang S, Nakayama S, Iino S, Tomita T. Voltage sensitivity of slow wave frequency in isolated circular muscle strips from guinea pig gastric antrum. Am J Physiol. 1999. 276:G518–G528.24. Sato K, Sanders KM, Gerthoffer WT, Publicover NG. Sources of calcium utilized in cholinergic responses in canine colonic smooth muscle. Am J Physiol. 1994. 267:C1666–C1673.
Article25. Koh SD, Sanders KM. Modulation of Ca2+ current in canine colonic myocytes by cyclic nucleotide-dependent mechanisms. Am J Physiol. 1996. 271:C794–C803.26. Xu WX, Kim SJ, Kim SJ, So I, Kang TM, Rhee JC, Kim KW. Effect of stretch on calcium channel currents recorded from the antral circular myocytes of guinea-pig stomach. Pflugers Arch. 1996. 432:159–164.
Article27. Kamimura N, Suga S, Wada J, Mio Y, Suzuki T, Wakui M. Excitatory and inhibitory actions of norepinephrine on the Ba2+ current through L-type Ca2+ channels of smooth muscle cells of guinea-pig vas deferens. J Cell Physiol. 1996. 169:373–379.28. Wade GR, Barbera J, Sims SM. Cholinergic inhibition of Ca2+ current in guinea-pig gastric and tracheal smooth muscle cells. J Physiol. 1996. 491:307–319.29. Huizinga JD, Thuneberg L, Kluppel M, Malysz J, Mikkelsen HB, Bernstein A. W/kit gene required for intestinal pacemaker activity. Nature. 1995. 373:347–349.30. Tomita T, Pang YW, Ogino K. The effects of nickel and cobalt ions on the spontaneous electrical activity, slow wave, in the circular muscle of the guinea-pig gastric antrum. J Smooth Muscle Res. 1998. 34:89–100.
Article31. Isenberg G, Klockner U. Calcium tolerant ventricular myocytes prepared by pre-incubation in a "K-B-medium". Pflugers Arch. 1982. 395:6–18.32. Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981. 391:85–100.33. Hille B. Ionic channels of excitable membranes. 1992. 2nd. Mass: Sinauer, Sunderland;457–462.34. Inoue Y, Xiong Z, Kitamura K, Kuriyama H. Modulation produced by nifedipine of the unitary Ba current of dispersed smooth muscle cells of the rabbit ileum. Pflugers Arch. 1989. 414:534–542.
Article35. Schoemaker H. Polyamines allosterically modulate [3H]nitrendipine binding to the voltage-sensitive calcium in rat brain. Eur J Pharmacol. 1992. 225:167–169.36. Hougaard DM, Fujiwara K, Larsson LI. Immunocytochemical localization of polyamines in normal and neoplastic cells. Comparisons to the formaldehyde-fluorescamine and o-phthalaldehyde methods. Histochem J. 1987. 19:643–650.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of ryanodine on the intracellular Na+ activity and tension and action potentials of rat and guinea pig cardiac ventricular muscles
- The Effects of Flumazenil and Verapamil on the Relaxation of Midazolam in Isolated Guinea-pig Tracheal Smooth Muscle
- The Effects of Heparin and Protamine on Contraction of Tracheal Smooth Muscle Induced by Carbachol in the Guinea Pig
- Muscarinic receptor subtype controlling the carbachol-induced muscle contraction in guinea pig gastric antrum
- Influence of the epithelium on the contraction of guinea pig isolated tracheal smooth muscle