J Korean Med Sci.
2006 Dec;21(6):1005-1011. 10.3346/jkms.2006.21.6.1005.
IL-2 Pathway Blocking in Combination with Anti-CD154 Synergistically Establishes Mixed Macrochimerism with Limited Dose of Bone Marrow Cells and Prolongs Skin Graft Survival in Mice
- Affiliations
-
- 1Department of Surgery, Hallym University College of Medicine, Chuncheon, Korea.
- 2Department of Surgery, Seoul National University College of Medicine, 28 Yongon-dong, Chongno-gu, Seoul, Korea. jwhamd@snu.ac.kr
- 3Transplantation Research Institute, Seoul National University Medical Research Center, Korea.
- 4Xenotransplantation Research Center, Seoul National University Hospital, Seoul, Korea.
- KMID: 1713109
- DOI: http://doi.org/10.3346/jkms.2006.21.6.1005
Abstract
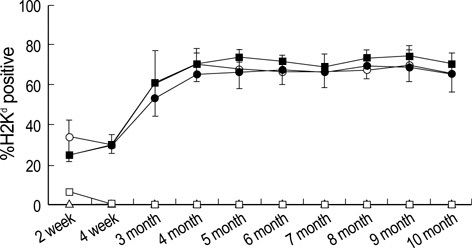
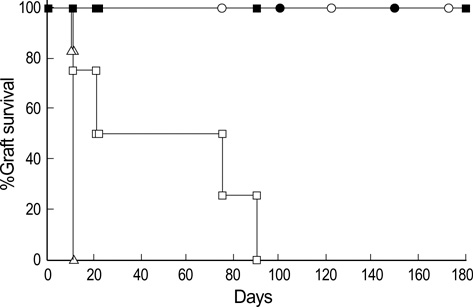
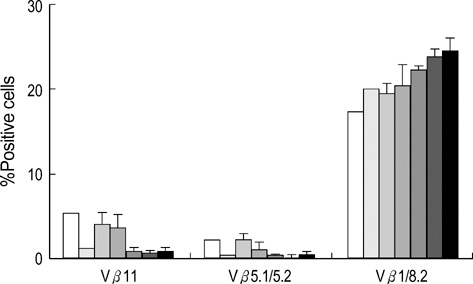
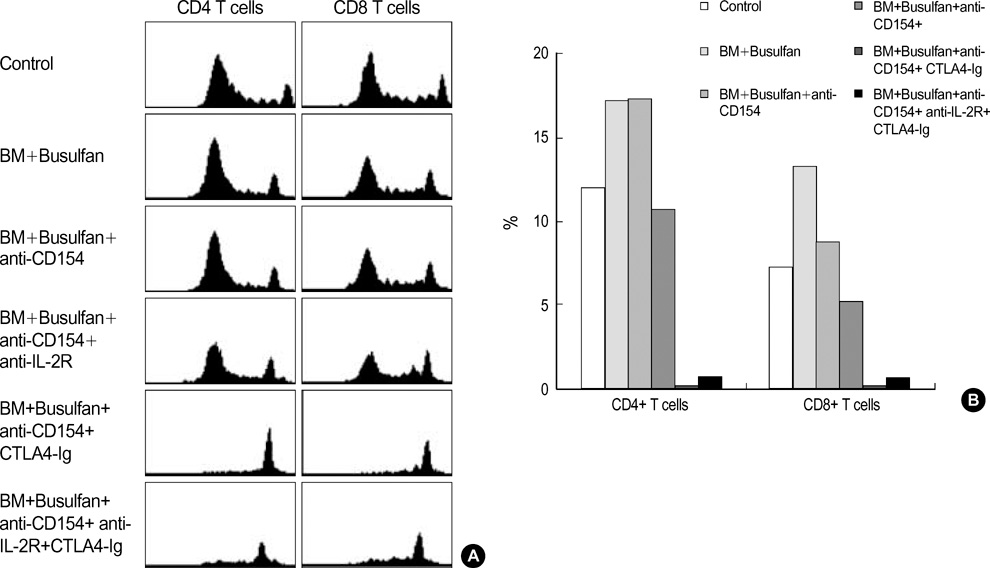
- To facilitate the establishment of mixed chimerism with limited dose of bone marrow (BM) cells, and to achieve tolerance in skin graft model, combined blocking of costimulatory pathway and IL-2 pathway was used in minimally myeloablative model using busulfan. BM cells (2.5 x 10(7)) of BALB/c were injected into C57BL/6 mice at day 0 with full thickness skin graft after single dose injection of busulfan (25 mg/kg) on day-1. Recipients were grouped and injected the anti-CD154, CTLA4-Ig, anti-IL-2R at days 0, 2, 4, and 6 according to protocol. Mixed macrochimerism were induced in groups treated with anti-CD154+anti-CTLA4-Ig, anti-CD154+anti-IL-2R, and anti-CD154+anti-CTLA4 Ig+anti-IL-2R. Three groups having chimerism enjoyed prolonged graft survival more than 6 months. Superantigen deletion study revealed deletion of alloreactive T cells in combined blockade treated groups. In graft versus host disease model using CFSE staining, CD4+ T cell and CD8+ T cell proliferation were reduced in groups treated with CTLA4-Ig or anti-IL-2R or both in combination with anti-CD154. However, anti-IL-2R was not so strong as CTLA4-Ig in terms of inhibition of T cell proliferation. In conclusion, IL-2 pathway blocking combined with anti-CD154 can establish macrochimerism with limited dose of BM transplantation and induce specific tolerance to allograft.
Keyword
MeSH Terms
Figure
Reference
-
1. Rossini AA, Greiner DL, Mordes JP. Induction of immunologic tolerance for transplantation. Physiol Rev. 1999. 79:99–141.
Article2. Uchida N, Shirasugi N, Akiyama Y, Matsumoto K, Shimazu M, Kitajima M, Hamano K, Aramaki O, Ikeda Y, Niimi M. Induction of indefinite survival of fully allogeneic cardiac grafts and generation of regulatory cells by intratracheal delivery of alloantigens under blockade of the CD40 pathway. Transplantation. 2003. 75:878–884.
Article3. Graca L, Honey K, Adams E, Cobbold SP, Waldmann H. Cutting Edge: Anti-CD154 therapeutic antibodies induce infectious transplantation tolerance. J Immunol. 2000. 165:4783–4786.
Article4. Niimi M, Pearson TC, Larsen CP, Alexander DZ, Hollenbaugh D, Aruffo A, Linsley PS, Thomas E, Campbell K, Fanslow WC, Geha RS, Morris PJ, Wood KJ. The role of the CD40 pathway in alloantigen-induced hyporesponsiveness in vivo. J Immunol. 1998. 161:5331–5337.5. Kenyon NS, Fernandez LA, Lehmann R, Masetti M, Ranuncoli A, Chatzipetrou M, Iaria G, Han D, Wagner JL, Ruiz P, Berho M, Inverardi L, Alejandro R, Mintz DH, Kirk AD, Harlan DM, Burkly LC, Ricordi C. Long-term survival and function of intrahepatic islet allografts in baboons treated with humanized anti-CD154. Diabetes. 1999. 48:1473–1481.
Article6. Elster EA, Xu H, Tadaki DK, Burkly LC, Berning JD, Baumgartner RE, Cruzata F, Patterson NB, Harlan DM, Kirk AD. Primate skin allotransplantation with anti-CD154 monotherapy. Transplant Proc. 2001. 33:675–676.
Article7. Kirk AD, Burkly LC, Batty DS, Baumgartner RE, Berning JD, Buchanan K, Fechner JH Jr, Germond RL, Kampen RL, Patterson NB, Swanson SJ, Tadaki DK, TenHoor CN, White L, Knechtle SJ, Harlan DM. Treatment with humanized monoclonal antibody against CD154 prevents acute renal allograft rejection in nonhuman primates. Nat Med. 1999. 5:686–693.
Article8. Sayegh MH, Zheng XG, Magee C, Hancock WW, Turka LA. Donor antigen is necessary for the prevention of chronic rejection in CTLA4Ig-treated murine cardiac allograft recipients. Transplantation. 1997. 64:1646–1650.9. Pearson TC, Alexander DZ, Winn KJ, Linsley PS, Lowry RP, Larsen CP. Transplantation tolerance induced by CTLA4-Ig. Transplantation. 1994. 57:1701–1706.10. Sayegh MH, Akalin E, Hancock WW, Russell ME, Carpenter CB, Linsley PS, Turka LA. CD28-B7 blockade after alloantigenic challenge in vivo inhibits Th1 cytokines but spares Th2. J Exp Med. 1995. 181:1869–1874.
Article11. Jones TR, Ha J, Williams MA, Adams AB, Durham MM, Rees PA, Cowan SR, Pearson TC, Larsen CP. The role of the IL-2 pathway in costimulation blockade-resistant rejection of allografts. J Immunol. 2002. 168:1123–1130.
Article12. Trambley J, Bingaman AW, Lin A, Elwood ET, Waitze SY, Ha J, Durham MM, Corbascio M, Cowan SR, Pearson TC, Larsen CP. Asialo GM1+ CD8+ T cells play a critical role in costimulation blockade-resistant allograft rejection. J Clin Invest. 1999. 104:1715–1722.
Article13. Guo Z, Meng L, Kim O, Wang J, Hart J, He G, Alegre ML, Thistlethwaite JR Jr, Pearson TC, Larsen CP, Newell KA. CD8 T cell-mediated rejection of intestinal allografts is resistant to inhibition of the CD40/CD154 cotimulatory pathway. Transplantation. 2001. 71:1351–1354.14. Kirkman RL, Barrett LV, Gaulton GN, Kelley VE, Ythier A, Strom TB. Administration of an anti-interleukin 2 receptor monoclonal antibody prolongs cardiac allograft survival in mice. J Exp Med. 1985. 162:358–362.
Article15. Kupiec-Weglinski JW, Diamantstein T, Tilney NL, Strom TB. Therapy with monoclonal antibody to interleukin 2 receptor spares suppressor T cells and prevents or reverses acute allograft rejection in rats. Proc Natl Acad Sci USA. 1986. 83:2624–2627.
Article16. Reed MH, Shapiro ME, Strom TB, Milford EL, Carpenter CB, Weinberg DS, Reimann KA, Letvin NL, Waldmann TA, Kirkman RL. Prolongation of primate renal allograft survival by anti-Tac, an anti-human IL-2 receptor monoclonal antibody. Transplantation. 1989. 47:55–59.
Article17. MacLean JA, Su Z, Guo Y, Sy MS, Colvin RB, Wong JT. Anti-CD3: anti-IL-2 receptor bispecific monoclonal antibody. Targeting of activated T cells in vitro. J Immunol. 1993. 150:1619–1628.18. Nakamura T, Good RA, Yasumizu R, Inoue S, Oo MM, Hamashima Y, Ikehara S. Successful liver allografts in mice by combination with allogeneic bone marrow transplantation. Proc Natl Acad Sci USA. 1986. 83:4529–4532.
Article19. Ikehara S, Ohtsuki H, Good RA, Asamoto H, Nakamura T, Sekita K, Muso E, Tochino Y, Ida T, Kuzuya H, Imura H, Hamashima Y. Prevention of type I diabetes in nonobese diabetic mice by allogenic bone marrow transplantation. Proc Natl Acad Sci USA. 1985. 82:7743–7747.
Article20. Adams AB, Durham MM, Kean L, Shirasugi N, Ha J, Williams MA, Rees PA, Cheung MC, Mittelstaedt S, Bingaman AW, Archer DR, Pearson TC, Waller EK, Larsen CP. Costimulation blockade, busulfan, and bone marrow promote titratable macrochimerism, induce transplantation tolerance, and correct genetic hemoglobinopathies with minimal myelosuppression. J Immunol. 2001. 167:1103–1111.
Article21. Durham MM, Bingaman AW, Adams AB, Ha J, Waitze SY, Pearson TC, Larsen CP. Cutting edge: administration of anti-CD40 ligand and donor bone marrow leads to hemopoietic chimerism and donor-specific tolerance without cytoreductive conditioning. J Immunol. 2000. 165:1–4.
Article22. Brodsky I, Bulova S, Crilley P. The role of busulfan/cyclophosphamide regimens in allogeneic and autologous bone marrow transplantation. Cancer Invest. 1989. 7:509–513.
Article23. Wekerle T, Kurtz J, Ito H, Ronquillo JV, Dong V, Zhao G, Shaffer J, Sayegh MH, Sykes M. Allogeneic bone marrow transplantation with co-stimulatory blockade induces macrochimerism and tolerance without cytoreductive host treatment. Nat Med. 2000. 6:464–469.
Article24. Adams AB, Shirasugi N, Jones TR, Williams MA, Durham MM, Ha J, Dong Y, Guo Z, Newell KA, Pearson TC, Larsen CP. Conventional immunosuppression is compatible with costimulation blockade-based, mixed chimerism tolerance induction. Am J Transplant. 2003. 3:895–901.
Article25. Ildstad ST, Sachs DH. Reconstitution with syngeneic plus allogeneic or xenogeneic bone marrow leads to specific acceptance of allografts or xenografts. Nature. 1984. 307:168–170.
Article26. Sharabi Y, Sachs DH. Mixed chimerism and permanent specific transplantation tolerance induced by a nonlethal preparative regimen. J Exp Med. 1989. 169:493–502.
Article27. Markees TG, Philips NE, Gordon EJ, Noelle RJ, Shultz LD, Mordes JP, Greiner DL, Rossini AA. Long-term survival of skin allografts induced by donor splenocytes and anti-CD154 antibody in thymectomized mice requires CD4+ T cells, interferon γ and CTLA4. J Clin Invest. 1998. 101:2446–2455.28. Tomita Y, Sachs DH, Sykes M. Myleosuppressive conditioning is required to achieve engraftment of pluripotent stem cells contained in moderate doses of syngeneic bone marrow. Blood. 1994. 83:939–948.29. Shehee WR, Oliver P, Smithies O. Lethal thalassemia after insertional disruption of the mouse major adult β-globin gene. Proc Natl Acad Sci USA. 1993. 90:3177–3181.30. Dai Z, Konieczny BT, Baddoura FK, Lakkis FG. Impaired alloantigen-mediated T cell apoptosis and failure to induce long-term allograft survival in IL-2-deficient mice. J Immunol. 1998. 161:1659–1663.31. Ferrara JL, Marion A, McIntyre JF, Murphy GF, Burakoff SJ. Amelioration of acute graft vs host disease due to minor histocompatibility antigens by in vivo administration of anti-interleukin 2 receptor antibody. J Immunol. 1986. 137:1874–1877.32. Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A, Bluestone JA. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000. 12:431–440.
Article33. Kumanogoh A, Wang X, Lee I, Watanabe C, Kamanaka M, Shi W, Yoshida K, Sato T, Habu S, Itoh M, Sakaguchi N, Sakaguchi S, Kikutani H. Increased T cell autoreactivity in the absence of CD40-CD40 ligand interactions: a role of CD40 in regulatory T cell development. J Immunol. 2001. 166:353–360.
Article34. Suzuki K, Nakajima H, Saito Y, Saito T, Leonard WJ, Iwamoto I. Janus kinase 3 (Jak3) is essential for common cytokine receptor gamma chain (gamma(c))-dependent signaling: comparative analysis of gamma(c), Jak3, and gamma(c) and Jak3 double-deficient mice. Int Immunol. 2000. 12:123–132.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Induction of Immune Tolerance by Macrochimerism: Preliminary Study for Overcome of Islet Allograft Rejection
- Alteration of Molecules and Cytokines Related to the Activation of T Lymphocyte in Immune Tolerance Induced Mice Model
- Role of Regulatory T Cells in Transferable Immunological Tolerance to Bone Marrow Donor in Murine Mixed Chimerism Model
- Platelets Induce Proliferation of Human Umbilical Vein Endothelial Cells via CD154-CD40 Pathway Independently of VEGF
- Two-signal blockade with anti-CD45RB and anti-CD154 monoclonal antibodies inhibits graft rejection via CD4-dependent mechanisms in allogeneic skin transplantation