Korean J Gastroenterol.
2013 Dec;62(6):359-364. 10.4166/kjg.2013.62.6.359.
Unconvincing Diagnosis of a Rare Subtype of Primary Gastric Lymphoma with Incongruent Endoscopic Presentation: A Case of Gastric Schwannoma
- Affiliations
-
- 1Department of Surgery, Kyungpook National University Hospital, Kyungpook National University School of Medicine, Daegu, Korea.
- 2Department of Surgery, Catholic University of Daegu School of Medicine, Daegu, Korea. ihkim@cu.ac.kr
- KMID: 1711292
- DOI: http://doi.org/10.4166/kjg.2013.62.6.359
Abstract
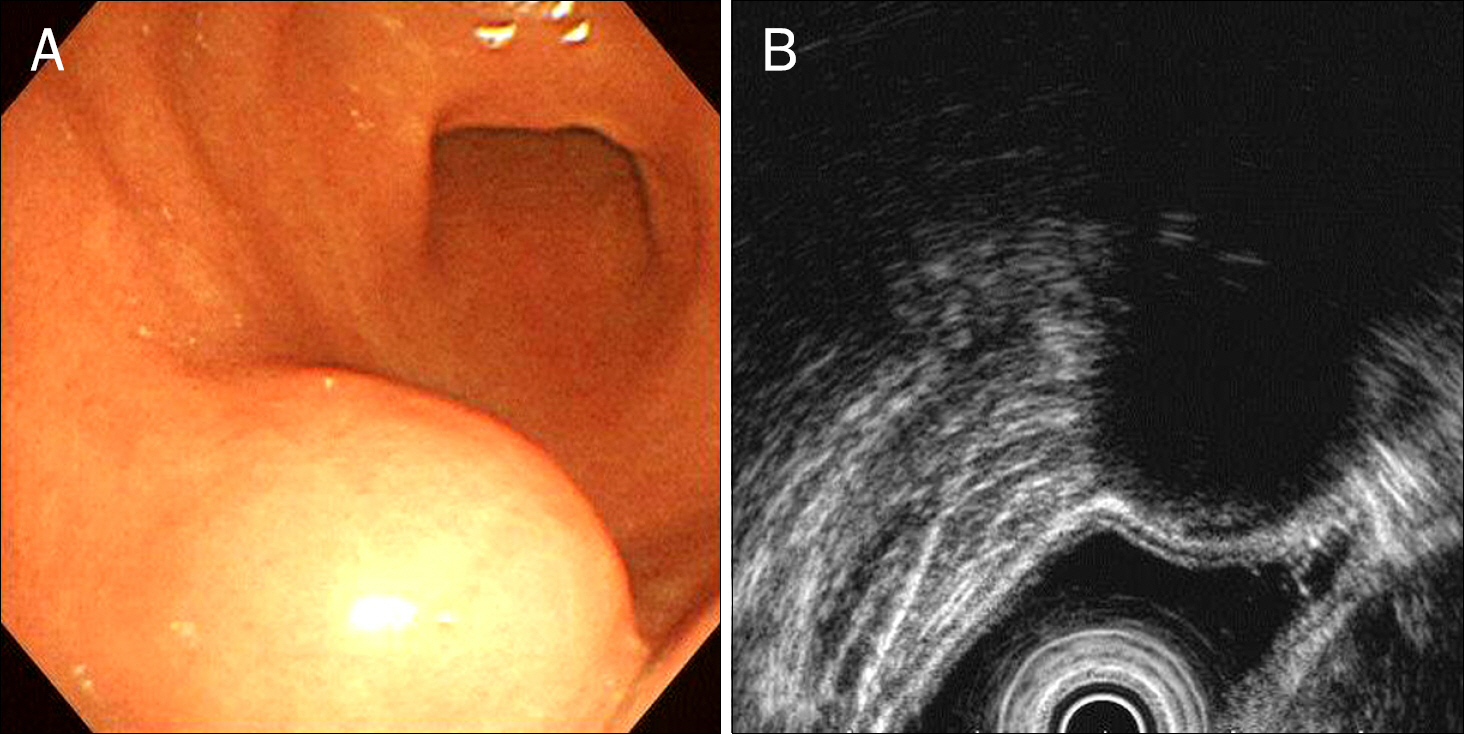
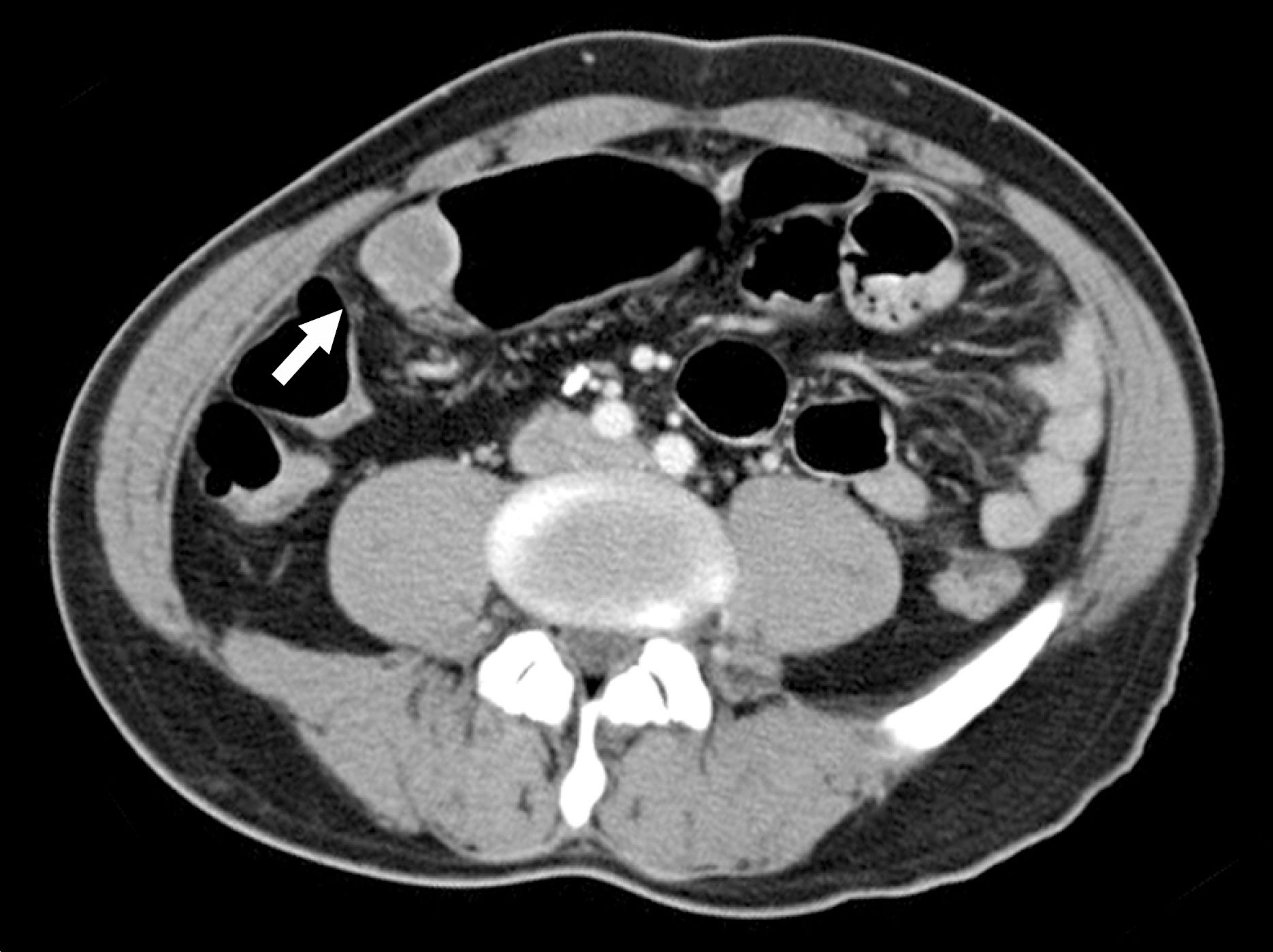
- Primary gastric lymphoma is a rare gastric malignancy. Its diagnostic process is complex. Clinician may find initial diagnosis of primary gastric lymphoma unreliable, especially when it indicates the rarest subtype of gastric lymphoma, while its initial endoscopic presentation fails to raise the slightest suspicion of primary gastric lymphoma. A 53-year-old Korean man was diagnosed, by endoscopic examination, with a round submucosal tumor of the stomach. Deep endoscopic biopsy, however, confirmed CD5 positive gastric lymphoma. Surgical treatment was performed for diagnosis and treatment. Postoperative histological examination confirmed gastric schwannoma. Gastric schwannoma is a spindle cell tumor, characterized by a peripheral cuff-like lymphocytic infiltration. Deep endoscopic biopsy may have been misdirected to the peripheral lymphoid cuff, failing to acquire spindle cells. The literature has been reviewed, and options for diagnostic accuracy have been suggested.
MeSH Terms
Figure
Reference
-
References
1. Ahmad A, Govil Y, Frank BB. Gastric mucosa-associated lymphoid tissue lymphoma. Am J Gastroenterol. 2003; 98:975–986.
Article2. Doglioni C, Ponzoni M, Ferreri AJ, Savio A. Gruppo Italiano Patologi Apparato Digerente (GIPAD); Società Italiana di Anatomia Patologica e Citopatologia Diagnostica/International Academy of Pathology, Italian division (SIAPEC/IAP). Gastric lymphoma: the histology report. Dig Liver Dis. 2011; 43(Suppl 4):S310–S318.
Article3. Jaso J, Chen L, Li S, et al. CD5-positive mucosa-associated lymphoid tissue (MALT) lymphoma: a clinicopathologic study of 14 cases. Hum Pathol. 2012; 43:1436–1443.
Article4. Pandey M, Wadhwa MK, Patel HP, Kothari KC, Shah M, Patel DD. Malignant lymphoma of the gastrointestinal tract. Eur J Surg Oncol. 1999; 25:164–167.
Article5. Kodera Y, Yamamura Y, Nakamura S, et al. The role of radical gastrectomy with systematic lymphadenectomy for the diagnosis and treatment of primary gastric lymphoma. Ann Surg. 1998; 227:45–50.
Article6. Koch P, del Valle F, Berdel WE, et al. German Multicenter Study Group. Primary gastrointestinal non-Hodgkin's lymphoma: II. Combined surgical and conservative or conservative management only in localized gastric lymphoma–results of the prospective German Multicenter Study GIT NHL 01/92. J Clin Oncol. 2001; 19:3874–3883.
Article7. Choi MK, Kim GH. Diagnosis and treatment of gastric MALT lymphoma. Korean J Gastroenterol. 2011; 57:272–280.
Article8. Voltaggio L, Murray R, Lasota J, Miettinen M. Gastric schwannoma: a clinicopathologic study of 51 cases and critical review of the literature. Hum Pathol. 2012; 43:650–659.
Article9. Kataoka M, Kawai T, Yagi K, et al. Mucosal cutting biopsy technique for histological diagnosis of suspected gastrointestinal stromal tumors of the stomach. Dig Endosc. 2013; 25:274–280.
Article10. Mekky MA, Yamao K, Sawaki A, et al. Diagnostic utility of EUSguided FNA in patients with gastric submucosal tumors. Gastrointest Endosc. 2010; 71:913–919.
Article11. Raber MH, Ziedses des Plantes CMP, Vink R, Klaase JM. Gastric schwannoma presenting as an incidentaloma on CT-scan and MRI. Gastroenterol Res. 2010; 3:276–280.
Article12. Lin CS, Hsu HS, Tsai CH, Li WY, Huang MH. Gastric schwannoma. J Chin Med Assoc. 2004; 67:583–586.