J Gynecol Oncol.
2014 Apr;25(2):124-129. 10.3802/jgo.2014.25.2.124.
Use of colony-stimulating factor in patients with ovarian cancer receiving paclitaxel and carboplatin in Japan
- Affiliations
-
- 1Department of Medical Oncology, Nippon Medical School Musashikosugi Hospital, Kawasaki, Japan. kharano@nms.ac.jp
- 2Center for Advanced Medicine and Clinical Research, Nagoya University Hospital, Nagoya, Japan.
- 3Global Health Consulting Japan Co. Ltd, Tokyo, Japan.
- KMID: 1708335
- DOI: http://doi.org/10.3802/jgo.2014.25.2.124
Abstract
OBJECTIVE
To assess the use of colony-stimulating factors (CSFs) in patients with ovarian cancer who receive adjuvant paclitaxel and carboplatin chemotherapy in clinical practice and to assess whether the frequency of CSF use differs among hospitals in Japan.
METHODS
CSF use in patients with ovarian cancer who received first-line paclitaxel and carboplatin was analyzed retrospectively using data from the Japanese hospitalization payment system.
RESULTS
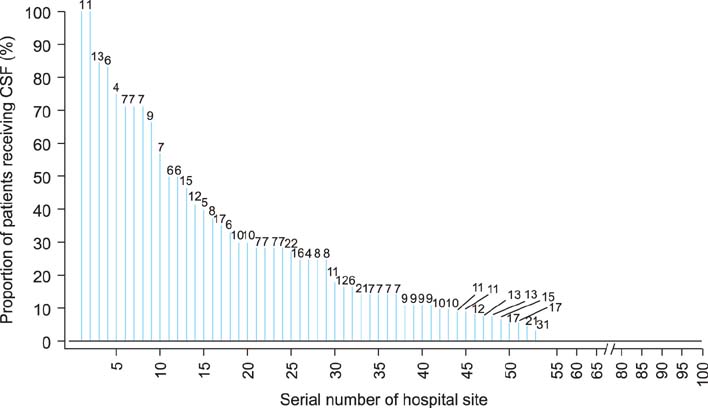
A total of 1,050 patients at 104 hospitals were identified. The median age was 60 years (range, 22 to 88 years). Of these, 163 patients (15.5%) were diagnosed with neutropenia and 134 patients (12.8%) received CSFs. Among the patients who received CSFs, 125 (93%) received them for the treatment of neutropenia without fever and 1 received them for febrile neutropenia. In total, CSFs were administered for 272 cycles of chemotherapy. Among them, CSFs were used as treatment for neutropenia without fever in 259 cycles (95%), as prophylaxis (primary or secondary) in 12 cycles (4%), and as treatment for febrile neutropenia in 1 cycle. Among hospitals, a median of 4.0% of patients received CSFs with an interquartile range of 25% (Q1, 0%; Q3, 25%). A logistic random effects model showed that the variation in the proportion of patients receiving CSFs among the 104 hospitals was 2.0 (p<0.001), suggesting that the use of CSFs varied across hospitals.
CONCLUSION
Most patients received CSFs for neutropenia without fever. Standardized and evidence-based use of CSFs is critically required among hospitals in Japan.
MeSH Terms
Figure
Reference
-
1. Smith TJ, Khatcheressian J, Lyman GH, Ozer H, Armitage JO, Balducci L, et al. 2006 update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guideline. J Clin Oncol. 2006; 24:3187–3205.2. Crawford J, Armitage J, Balducci L, Becker PS, Blayney DW, Cataland SR, et al. NCCN clinical practice guidelines in oncology: Myeloid growth factors. ver. 2 [Internet]. Fort Washington, PA: National Comprehensive Cancer Network;2013. cited 2013 Nov 29. Available from: http://www.nccn.org/professionals/physician_gls/pdf/myeloid_growth.pdf.3. Aapro MS, Bohlius J, Cameron DA, Dal Lago L, Donnelly JP, Kearney N, et al. 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer. 2011; 47:8–32.4. Hartmann LC, Tschetter LK, Habermann TM, Ebbert LP, Johnson PS, Mailliard JA, et al. Granulocyte colony-stimulating factor in severe chemotherapy-induced afebrile neutropenia. N Engl J Med. 1997; 336:1776–1780.5. Garcia-Carbonero R, Mayordomo JI, Tornamira MV, Lopez-Brea M, Rueda A, Guillem V, et al. Granulocyte colony-stimulating factor in the treatment of high-risk febrile neutropenia: a multicenter randomized trial. J Natl Cancer Inst. 2001; 93:31–38.6. Ramsey SD, McCune JS, Blough DK, McDermott CL, Clarke L, Malin JL, et al. Colony-stimulating factor prescribing patterns in patients receiving chemotherapy for cancer. Am J Manag Care. 2010; 16:678–686.7. Potosky AL, Malin JL, Kim B, Chrischilles EA, Makgoeng SB, Howlader N, et al. Use of colony-stimulating factors with chemotherapy: opportunities for cost savings and improved outcomes. J Natl Cancer Inst. 2011; 103:979–982.8. Bennett CL, Djulbegovic B, Norris LB, Armitage JO. Colony-stimulating factors for febrile neutropenia during cancer therapy. N Engl J Med. 2013; 368:1131–1139.9. Katsumata N, Yasuda M, Takahashi F, Isonishi S, Jobo T, Aoki D, et al. Dose-dense paclitaxel once a week in combination with carboplatin every 3 weeks for advanced ovarian cancer: a phase 3, open-label, randomised controlled trial. Lancet. 2009; 374:1331–1338.10. du Bois A, Luck HJ, Meier W, Adams HP, Mobus V, Costa S, et al. A randomized clinical trial of cisplatin/paclitaxel versus carboplatin/paclitaxel as first-line treatment of ovarian cancer. J Natl Cancer Inst. 2003; 95:1320–1329.11. Ministry of Health, Labor, and Welfare. Overview of revision of payment system related to DPC 2012 [Internet]. Tokyo: Ministry of Health, Labor, and Welfare;cited 2013 Jun 29. Available from: http://www.mhlw.go.jp/bunya/iryouhoken/iryouhoken15/dl/h24_01-05.pdf.12. Mahoney FI, Barthel DW. Functional evaluation: the barthel index. Md State Med J. 1965; 14:61–65.13. Ozols RF, Bundy BN, Greer BE, Fowler JM, Clarke-Pearson D, Burger RA, et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2003; 21:3194–3200.14. Alvarez Secord A, Bae-Jump V, Havrilesky LJ, Calingaert B, Clarke-Pearson DL, Soper JT, et al. Attitudes regarding the use of hematopoietic colony-stimulating factors and maintenance of relative dose intensity among gynecologic oncologists. Int J Gynecol Cancer. 2009; 19:447–454.15. Pharmaceuticals and Medical Devices Agency. GRAN SYRINGE: usage and dosage/efficacy or effectiveness [Internet]. Tokyo: Pharmaceuticals and Medical Devices Agency;2013. cited 2013 Jun 29. Available from: http://www.info.pmda.go.jp/go/pack/3399405A1027_4_06/.16. Vernooij F, Heintz P, Witteveen E, van der Graaf Y. The outcomes of ovarian cancer treatment are better when provided by gynecologic oncologists and in specialized hospitals: a systematic review. Gynecol Oncol. 2007; 105:801–812.17. Chan JK, Kapp DS, Shin JY, Husain A, Teng NN, Berek JS, et al. Influence of the gynecologic oncologist on the survival of ovarian cancer patients. Obstet Gynecol. 2007; 109:1342–1350.18. Vernooij F, Heintz AP, Coebergh JW, Massuger LF, Witteveen PO, van der Graaf Y. Specialized and high-volume care leads to better outcomes of ovarian cancer treatment in the Netherlands. Gynecol Oncol. 2009; 112:455–461.19. Ministry of Health, Labor, and Welfare. Agenda, compensation survey and DPC evaluation subcommittee 9th FY 2011 [Internet]. Tokyo: Ministry of Health, Labor, and Welfare;cited 2013 Jun 29. Available from: http://www.mhlw.go.jp/stf/shingi/2r9852000001u23a.html.20. Uramoto H, Kabashima M, Yamazaki K, Kadota T, Narimatsu M, Iwashige A, et al. What do cancer chemotherapy outpatients want?: results of a questionnaire survey. Gan To Kagaku Ryoho. 2006; 33:1681–1683.21. Jalali R, Dutta D, Kamble R, Gupta T, Munshi A, Sarin R, et al. Prospective assessment of activities of daily living using modified Barthel's Index in children and young adults with low-grade gliomas treated with stereotactic conformal radiotherapy. J Neurooncol. 2008; 90:321–328.22. Gazzotti MR, Malheiros SM, Batan Alith M, Nascimento O, Santoro IL, Jardim JR, et al. Quality of life and physical limitations in primary brain tumor patients. Qual Life Res. 2011; 20:1639–1643.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Recurrent Cervical Cancer Responded to Paclitaxel and Carboplatin Combination
- A case of neoadjuvant chemotherapy with taxol / carboplatin in advanced epithelial ovarian cancer
- Phase I Clinical Trial of Paclitaxel Plus Ifosfamide for the Patients with Refractory Ovarian Cancer
- Weekly versus 3-weekly paclitaxel in combination with carboplatin in advanced ovarian cancer: which is the optimal adjuvant chemotherapy regimen?
- Second-line Chemotherapy with Paclitaxel and Carboplatin for Patients with Recurrent Ovarian Carcinoma