Korean J Radiol.
2013 Jun;14(3):395-399. 10.3348/kjr.2013.14.3.395.
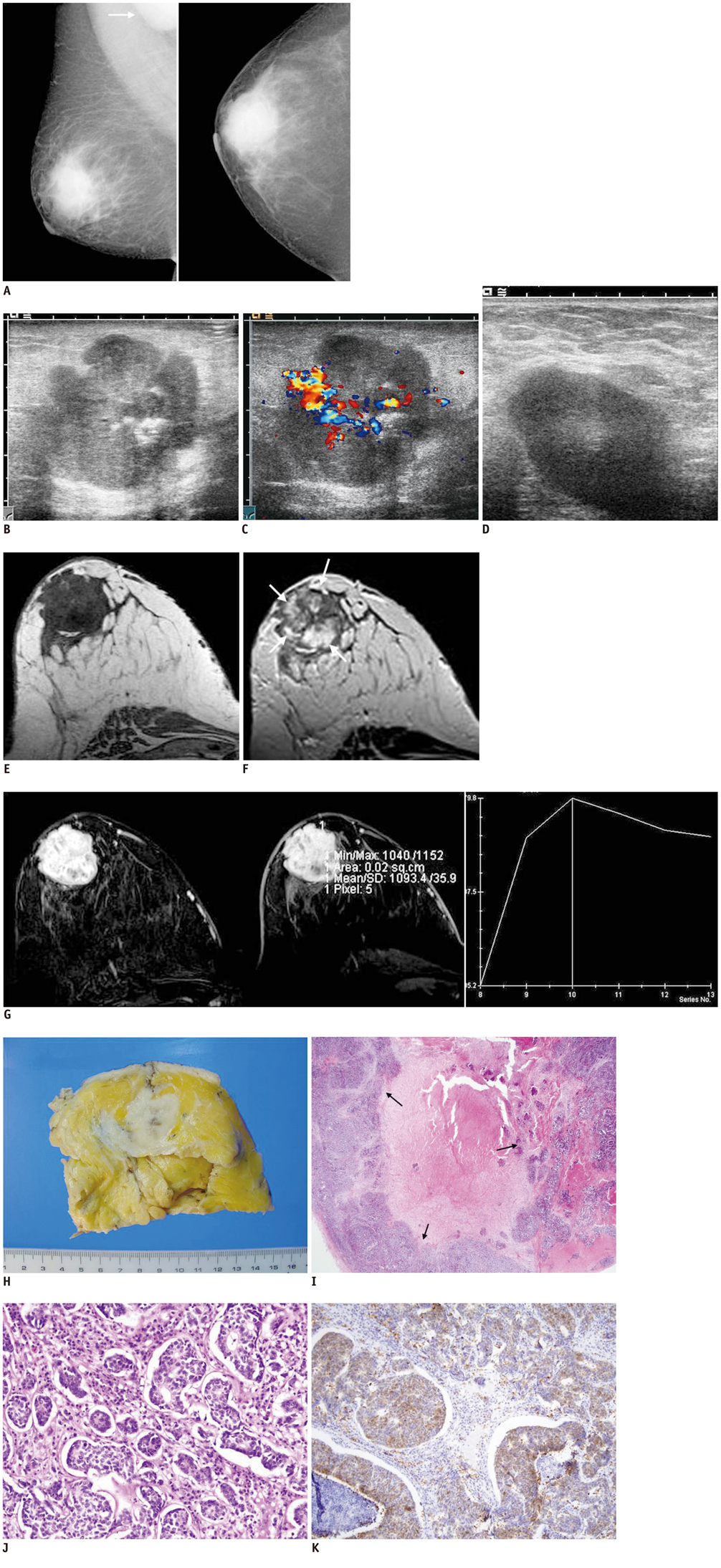
Primary Neuroendocrine Tumor of the Breast: Imaging Features
- Affiliations
-
- 1Department of Clinical Pathology, Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Uijeongbu 480-717, Korea.
- 2Department of Radiology, Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Uijeongbu 480-717, Korea. tiger@catholic.ac.kr
- 3Department of Surgery, Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Uijeongbu 480-717, Korea.
- KMID: 1705449
- DOI: http://doi.org/10.3348/kjr.2013.14.3.395
Abstract
- Focal neuroendocrine differentiation can be found in diverse histological types of breast tumors. However, the term, neuroendocrine breast tumor, indicates the diffuse expression of neuroendocrine markers in more than 50% of the tumor cell population. The imaging features of neuroendocrine breast tumor have not been accurately described due to extreme rarity of this tumor type. We present a case of a pathologically confirmed, primary neuroendocrine breast tumor in a 42-year-old woman, with imaging findings difficult to be differentiated from that of invasive ductal carcinoma.
MeSH Terms
Figure
Reference
-
1. Günhan-Bilgen I, Zekioglu O, Ustün EE, Memis A, Erhan Y. Neuroendocrine differentiated breast carcinoma: imaging features correlated with clinical and histopathological findings. Eur Radiol. 2003. 13:788–793.2. Mariscal A, Balliu E, Díaz R, Casas JD, Gallart AM. Primary oat cell carcinoma of the breast: imaging features. AJR Am J Roentgenol. 2004. 183:1169–1171.3. Kitakata H, Yasumoto K, Sudo Y, Minato H, Takahashi Y. A case of primary small cell carcinoma of the breast. Breast Cancer. 2007. 14:414–419.4. Ogawa H, Nishio A, Satake H, Naganawa S, Imai T, Sawaki M, et al. Neuroendocrine tumor in the breast. Radiat Med. 2008. 26:28–32.5. Irshad A, Ackerman SJ, Pope TL, Moses CK, Rumboldt T, Panzegrau B. Rare breast lesions: correlation of imaging and histologic features with WHO classification. Radiographics. 2008. 28:1399–1414.6. Zhang JY, Chen WJ. Bilateral primary breast neuroendocrine carcinoma in a young woman: report of a case. Surg Today. 2011. 41:1575–1578.7. An JK, Woo JJ, Kang JH, Kim EK. Small-cell neuroendocrine carcinoma of the breast. J Korean Surg Soc. 2012. 82:116–119.8. Maluf HM, Koerner FC. Carcinomas of the breast with endocrine differentiation: a review. Virchows Arch. 1994. 425:449–457.9. Sapino A, Righi L, Cassoni P, Papotti M, Pietribiasi F, Bussolati G. Expression of the neuroendocrine phenotype in carcinomas of the breast. Semin Diagn Pathol. 2000. 17:127–137.10. Tavassoli FA, Devilee P. Tavassoli FA, Devilee P, editors. Tumours of the breast. Pathology and genetics of tumours of the breast and female genital organs. World Health Organization Classification of Tumours Series. 2003. Lyon: IARC Press;32–34.11. Adegbola T, Connolly CE, Mortimer G. Small cell neuroendocrine carcinoma of the breast: a report of three cases and review of the literature. J Clin Pathol. 2005. 58:775–778.12. Sapino A, Righi L, Cassoni P, Papotti M, Gugliotta P, Bussolati G. Expression of apocrine differentiation markers in neuroendocrine breast carcinomas of aged women. Mod Pathol. 2001. 14:768–776.13. Yamaguchi R, Furusawa H, Nakahara H, Inomata M, Namba K, Tanaka M, et al. Clinicopathological study of invasive ductal carcinoma with large central acellular zone: special reference to magnetic resonance imaging findings. Pathol Int. 2008. 58:26–30.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Primary Breast Carcinoma with Neuroendocrine Features: Imaging Features on Mammography and Ultrasonography
- Primary Small Cell Neuroendocrine Carcinoma of the Breast: A Case Report With Literature Review
- Mammographic, Sonographic, and MRI Features of Primary Neuroendocrine Carcinoma of the Breast: A Case Report
- Small-cell neuroendocrine carcinoma of the breast
- Primary Neuroendocrine Carcinoma of the Breast: A Case Report and Literature Review