Cancer Res Treat.
2005 Apr;37(2):133-135.
Carboxypeptidase-G2 Rescue in a Patient with High Dose Methotrexate-induced Nephrotoxicity
- Affiliations
-
- 1Department of Pediatrics, Seoul National University College of Medicine, Seoul, Korea. hsahn@snu.ac.kr
- 2Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea.
Abstract
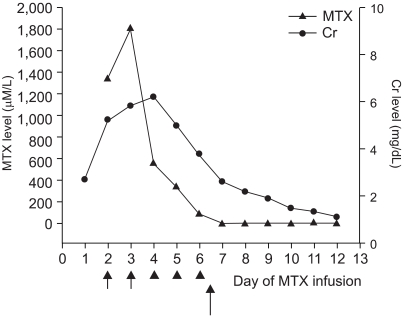
- A 13 year-old girl with osteosarcoma and pulmonary tumor recurrence developed acute renal failure following high dose methotrexate (12 g/m2) therapy, she had previously tolerated high dose methotrexate and her renal and hepatic functions were normal. Briefly, 48 hours after beginning methotrexate infusion her methotrexate concentration and creatinine level were 1338.8microM/L and 5.8 mg/dl, respectively. Grade IV oral mucositis and neutropenia with fever developed at 144 hours after MTX infusion. Hydration and alkalinization were continued and leucovorin rescue was intensified based on the plasma MTX concentrations. Plasma exchange was performed twice and hemodialysis 3 times without problems, but methotraxate and creatinine levels remained high, 91.9 microM/L, and 2.5 mg/dl, respectively. After 3 courses of hemodialysis carboxypeptidase-G2 (CPDG2) was administered at 50 U/kg, intravenously over 5 minutes. After 15 minutes of CPDG2 (Voraxaze(TM)) infusion, her plasma MTX concentration was 0.91microM/L and no rebound elevation or side effects developed. Thirteen days post-MTX infusion her renal function had normalized. We report here our experience of a dramatic methotrexate level reduction caused by CPDG2 administration.
MeSH Terms
Figure
Reference
-
1. Lankelma J, van der Klein E, Ramaekers F. The role of 7-hydroxymethotrexate during methotrexate anti-cancer therapy. Cancer Lett. 1980; 9:133–142. PMID: 7189691.
Article2. Bleyer WA. Methotrexate: clinical pharmacology, current status and therapeutic guidelines. Cancer Treat Rev. 1977; 4:87–101. PMID: 329989.
Article3. Flombaum CD, Meyers PA. High-dose leucovorin as sole therapy for methotrexate toxicity. J Clin Oncol. 1999; 17:1589–1594. PMID: 10334548.
Article4. Relling MV, Stapleton FB, Ochs J, Jones DP, Meyer W, Wainer IW, et al. Removal of methotrexate, leucovorin, and their metabolites by combined hemodialysis and hemoperfusion. Cancer. 1988; 62:884–888. PMID: 3261621.
Article5. Kawabata K, Makino H, Nagake Y, Tokioka H, Matsumi M, Morita Y, et al. A case of methotrexate-induced acute renal failure successfully treated with plasma perfusion and sequential hemodialysis. Nephron. 1995; 71:233–234. PMID: 8569964.6. Minton NP, Atkinson T, Sherwood RF. Molecular cloning of the Pseudomonas carboxypeptidase G2 gene and its expression in Escherichia coli and Pseudomonas putida. J Bacteriol. 1983; 156:1222–1227. PMID: 6358192.7. Widemann BC, Sung E, Anderson L, Salzer WL, Balis FM, Monitjo KS, et al. Pharmacokinetics and metabolism of the methotrexate metabolite, 2,4-diamino-N10-methylpteroic acid. J Pharmacol Exp Ther. 2000; 294:894–901. PMID: 10945838.8. Rosen G, Caparros B, Huvos AG, Kosloff C, Nirenberg A, Cacavio A, et al. Preoperative chemotherapy for osteogenic sarcoma: selection of postoperative adjuvant chemotherapy based on the response of the primary tumor to preoperative chemotherapy. Cancer. 1982; 49:1221–1230. PMID: 6174200.9. Widemann BC, Balis FM, Kempf-Bielack B, Bielack S, Pratt CB, Ferrari S, et al. High-dose methotrexate-induced nephrotoxicity in patients with osteosarcoma. Cancer. 2004; 100:2222–2232. PMID: 15139068.
Article10. Sohn JH, Rha SY, Jeung HC, Shin HJ, Goo YS, Chung HC, et al. Efficacy of pre- and postoperative chemotherapy in patients with osteosarcoma of the extremities. Cancer Res Treat. 2001; 33:520–526.
Article11. Kim PS, Ko YH, Jung JE, Kim CW, Cho SG, Kim HK, et al. A case of high dose methotrexate-induced acute renal failure successfully treated with plasma exchange and hemodialysis. Korean J Hematol. 2000; 35:58–61.12. Greil J, Wyss PA, Ludwig K, Bonakdar S, Scharf J, Beck JD, et al. Continuous plasma resin perfusion for detoxification of methotrexate. Eur J Pediatr. 1997; 156:533–536. PMID: 9243235.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of High Methotrexate-induced Acute Renal Failure Successfully Treated with Plasma Exchange and Hemodialysis
- The Effect of a High Dose of Methotrexate on Type II Collagen induced Arthritis in Rats
- Total Plasma Exchange in a Patient with HD-MTX-induced Acute Renal Failure: A Case Report
- Plasma Exchange in a Patient with a High Serum Methotrexate Level after High Dose-MTX Chemotherapy
- Pathological responses to preoperative high-dose methotrexate chemotherapy in osteosarcoma: experience in Korea cancer hospital