Korean J Ophthalmol.
2013 Apr;27(2):109-115. 10.3341/kjo.2013.27.2.109.
Capillary-free Vascularized Retina in Patients with Aggressive Posterior Retinopathy of Prematurity and Late Retinal Capillary Formation
- Affiliations
-
- 1Department of Ophthalmology, Seoul National University College of Medicine, Seoul, Korea. ysyu@snu.ac.kr
- 2Seoul Artificial Eye Center, Clinical Research Institute, Seoul National University Hospital, Seoul, Korea.
- KMID: 1503802
- DOI: http://doi.org/10.3341/kjo.2013.27.2.109
Abstract
- PURPOSE
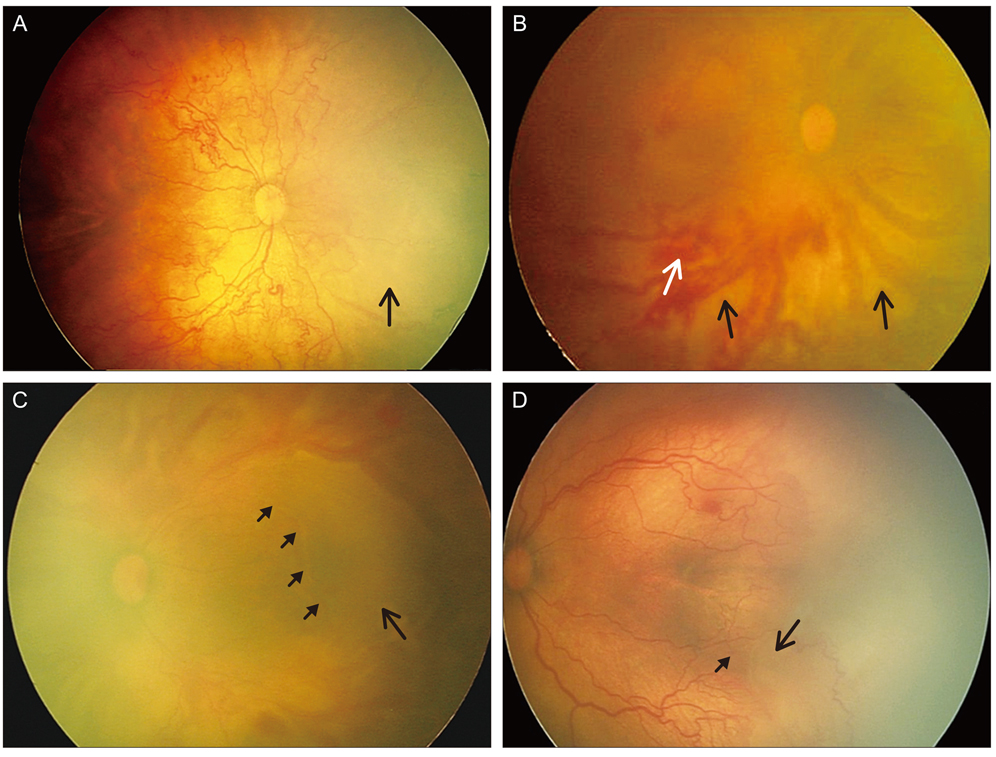
To report the clinical features, clinical course, and treatment outcomes after laser photocoagulation in infants with aggressive posterior retinopathy of prematurity (APROP) and capillary-free zones in vascularized retina.
METHODS
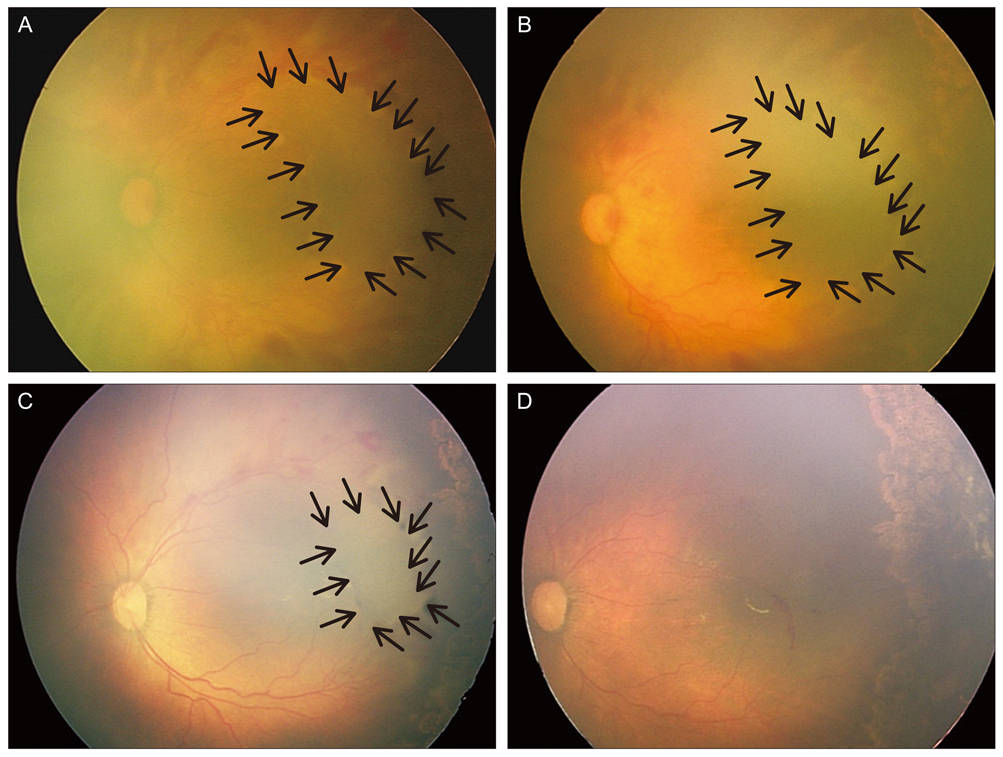
Six patients (12 eyes) with APROP and capillary-free zones in vascularized retina were retrospectively reviewed. Twelve eyes of six infants were included and were treated with laser photocoagulation for avascular retina and for capillary-free zones in vascularized retina, except for the posterior pole, and fundus findings were photographically-documented in sequence. In addition, anatomic and visual outcomes were evaluated with complications of APROP.
RESULTS
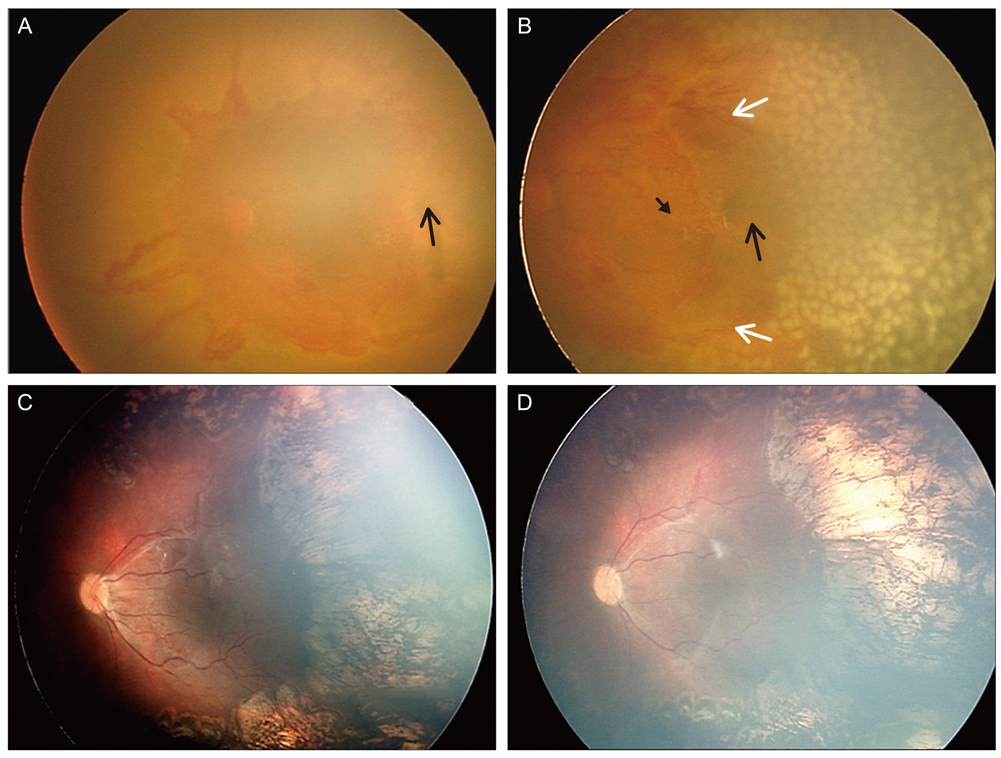
Among all of the consecutive infants with APROP, capillary-free zones in vascularized retina were demonstrated in 24% of the infants. All of the infants were >27 weeks of gestation age and had birth weights >1,000 g. After laser treatment, 7 eyes (58.3%) had favorable outcomes, and late capillary filling in capillary-free zones of vascularized retina were noted, however 4 eyes (33.3%) progressed to retinal detachment and 1 eye (8.3%) was complicated by a retinal fold-distorting posterior pole. The visual outcomes were associated with anatomic outcomes.
CONCLUSIONS
The anatomic outcomes in infants with APROP who had capillary-free zones were comparable to previously reported infants with APROP. The late capillary filling of capillary-free zones in vascularized retina was noted, and angiogenesis was considered to be involved. This process toward normal capillary formation or neovascularization in APROP, might determine its outcome.
MeSH Terms
Figure
Reference
-
1. International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity revisited. Arch Ophthalmol. 2005. 123:991–999.2. Early Treatment for Retinopathy of Prematurity Cooperative Group. Revised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol. 2003. 121:1684–1694.3. Morizane H. Initial sign and clinical course of the most severe form of acute proliferative retrolental fibroplasia (type II). Nihon Ganka Gakkai Zasshi. 1976. 80:54–61.4. Nissenkorn I, Kremer I, Gilad E, et al. 'Rush' type retinopathy of prematurity: report of three cases. Br J Ophthalmol. 1987. 71:559–562.5. Drenser KA, Trese MT, Capone A Jr. Aggressive posterior retinopathy of prematurity. Retina. 2010. 30:4 Suppl. S37–S40.6. Vinekar A, Trese MT, Capone A Jr. Photographic Screening for Retinopathy of Prematurity (PHOTO-ROP) Cooperative Group. Evolution of retinal detachment in posterior retinopathy of prematurity: impact on treatment approach. Am J Ophthalmol. 2008. 145:548–555.7. Yokoi T, Hiraoka M, Miyamoto M, et al. Vascular abnormalities in aggressive posterior retinopathy of prematurity detected by fluorescein angiography. Ophthalmology. 2009. 116:1377–1382.8. Khwarg SI, Yu HG, Yu YS. The outcome of cryotherapy for retinopathy of prematurity (ROP) according to ROP location. Korean J Ophthalmol. 1996. 10:92–96.9. Schulenburg WE, Tsanaktsidis G. Variations in the morphology of retinopathy of prematurity in extremely low birthweight infants. Br J Ophthalmol. 2004. 88:1500–1503.10. Section on Ophthalmology American Academy of Pediatrics. American Academy of Ophthalmology. American Association for Pediatric Ophthalmology and Strabismus. Screening examination of premature infants for retinopathy of prematurity. Pediatrics. 2006. 117:572–576.11. Azuma N, Ishikawa K, Hama Y, et al. Early vitreous surgery for aggressive posterior retinopathy of prematurity. Am J Ophthalmol. 2006. 142:636–643.12. Flynn JT, Chan-Ling T. Retinopathy of prematurity: two distinct mechanisms that underlie zone 1 and zone 2 disease. Am J Ophthalmol. 2006. 142:46–59.13. Lutty GA, Chan-Ling T, Phelps DL, et al. Proceedings of the third International Symposium on Retinopathy of Prematurity: an update on ROP from the lab to the nursery (November 2003, Anaheim, California). Mol Vis. 2006. 12:532–580.14. Mantagos IS, Vanderveen DK, Smith LE. Emerging treatments for retinopathy of prematurity. Semin Ophthalmol. 2009. 24:82–86.15. Kychenthal A, Dorta P, Katz X. Zone I retinopathy of prematurity: clinical characteristics and treatment outcomes. Retina. 2006. 26:7 Suppl. S11–S15.16. Trigler L, Weaver RG Jr, O'Neil JW, et al. Case series of angle-closure glaucoma after laser treatment for retinopathy of prematurity. J AAPOS. 2005. 9:17–21.17. Chen J, Smith LE. Retinopathy of prematurity. Angiogenesis. 2007. 10:133–140.18. Romagnoli C. Risk factors and growth factors in ROP. Early Hum Dev. 2009. 85:10 Suppl. S79–S82.19. Alon T, Hemo I, Itin A, et al. Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat Med. 1995. 1:1024–1028.20. Ashton N, Ward B, Serpell G. Effect of oxygen on developing retinal vessels with particular reference to the problem of retrolental fibroplasia. Br J Ophthalmol. 1954. 38:397–432.21. Claxton S, Fruttiger M. Role of arteries in oxygen induced vaso-obliteration. Exp Eye Res. 2003. 77:305–311.22. Gu X, El-Remessy AB, Brooks SE, et al. Hyperoxia induces retinal vascular endothelial cell apoptosis through formation of peroxynitrite. Am J Physiol Cell Physiol. 2003. 285:C546–C554.23. Pierce EA, Foley ED, Smith LE. Regulation of vascular endothelial growth factor by oxygen in a model of retinopathy of prematurity. Arch Ophthalmol. 1996. 114:1219–1228.24. Chan-Ling T, Page MP, Gardiner T, et al. Desmin ensheathment ratio as an indicator of vessel stability: evidence in normal development and in retinopathy of prematurity. Am J Pathol. 2004. 165:1301–1313.25. Hughes S, Chan-Ling T. Characterization of smooth muscle cell and pericyte differentiation in the rat retina in vivo. Invest Ophthalmol Vis Sci. 2004. 45:2795–2806.26. Hughes S, Yang H, Chan-Ling T. Vascularization of the human fetal retina: roles of vasculogenesis and angiogenesis. Invest Ophthalmol Vis Sci. 2000. 41:1217–1228.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- An Ultrastructural Study on the Early Morphologic Changes of the Retina in Streptozotocin-induced Diabetic Rats
- Solitary Retinal Capillary Hemangioma Treated with Cryotherapy
- Two Types of Retinal Capillary Path in Humans using Fluorescein Leukocyte Angiography
- Retinopathy of prematurity-mimicking retinopathy in full-term babies
- Three Cases of Retinal Capillary Hemangioma Presenting with Retinal Detachment