Obstet Gynecol Sci.
2013 Nov;56(6):368-374. 10.5468/ogs.2013.56.6.368.
Hepatitis A virus infection during pregnancy in Korea: Hepatitis A infection on pregnant women
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Korea University Guro Hospital, Korea University College of Medicine, Seoul, Korea. mjohmd@korea.ac.kr
- 2Department of Obstetrics and Gynecology, Inha University College of Medicine, Incheon, Korea.
- 3Division of Gastroenterology and Hepatology, Department of Internal Medicine, Korea University Guro Hospital, Korea University College of Medicine, Seoul, Korea.
- 4Department of Obstetrics and Gynecology, Kyung Hee University College of Medicine, Seoul, Korea.
- KMID: 1500370
- DOI: http://doi.org/10.5468/ogs.2013.56.6.368
Abstract
OBJECTIVE
Although there is a large body of data on acute hepatitis A virus (HAV) worldwide, data regarding the occurrence of HAV during pregnancy is limited. It is commonly acknowledged that HAV is not associated with severe outcomes or complications during pregnancy. In contrast, there are several reported cases of vertical HAV transmission. Moreover, it has been recently reported that HAV infection during pregnancy is associated with gestational complications. In Korea, the incidence of HAV infection has increased from 317 cases in 2002 to 13,117 cases in 2009. However, HAV infection during pregnancy is rarely reported in Korea.
METHODS
This study was conducted as a retrospective cohort series of pregnant women presenting to Korea University Medical Center between January 2000 and October 2009 in whom a diagnosis of HAV infection was made.
RESULTS
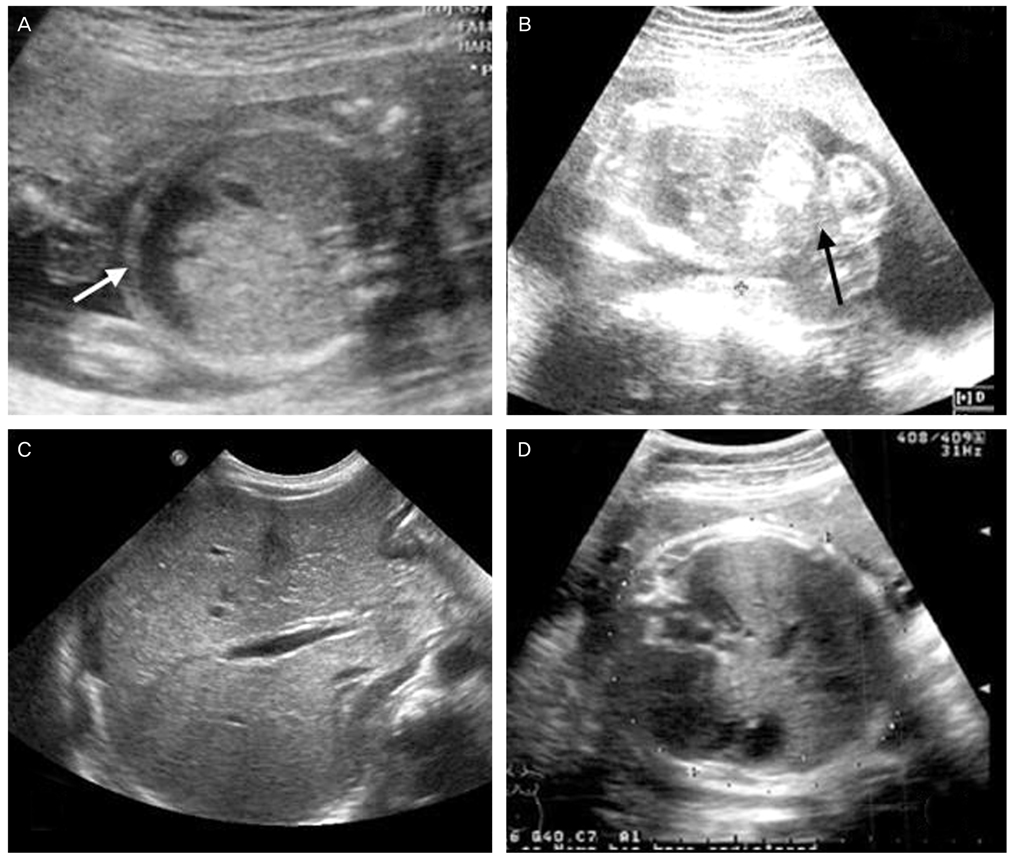
During study period, there were 12 cases of HAV in pregnant women, including two cases with preterm contraction, two cases with cholestatic hepatitis, and one case with fetal ascites and intra-abdominal calcification.
CONCLUSION
HAV infection during pregnancy is associated with high prevalence of maternal and fetal complications. Because the incidence of HAV infection in women of reproductive age is increasing, a further focus on preventing HAV infection during pregnancy is warranted.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Vaccination in pregnancy
Min-Jeong Oh
J Korean Med Assoc. 2016;59(7):523-528. doi: 10.5124/jkma.2016.59.7.523.Seroepidemiology of Hepatitis Viruses and Hepatitis B Genotypes of Female Marriage Immigrants in Korea
Jae-Cheol Kwon, Hye Young Chang, Oh Young Kwon, Ji Hoon Park, In Soo Oh, Hyung Joon Kim, Jun Hyung Lee, Ha-Jung Roh, Hyun Woong Lee
Yonsei Med J. 2018;59(9):1072-1078. doi: 10.3349/ymj.2018.59.9.1072.
Reference
-
1. Kemmer NM, Miskovsky EP. Hepatitis A. Infect Dis Clin North Am. 2000; 14:605–615.2. FitzSimons D, Hendrickx G, Vorsters A, Van Damme P. Hepatitis A and E: update on prevention and epidemiology. Vaccine. 2010; 28:583–588.3. Elinav E, Ben-Dov IZ, Shapira Y, Daudi N, Adler R, Shouval D, et al. Acute hepatitis A infection in pregnancy is associated with high rates of gestational complications and preterm labor. Gastroenterology. 2006; 130:1129–1134.4. Jacobsen KH, Koopman JS. Declining hepatitis A seroprevalence: a global review and analysis. Epidemiol Infect. 2004; 132:1005–1022.5. Cuthbert JA. Hepatitis A: old and new. Clin Microbiol Rev. 2001; 14:38–58.6. Tong MJ, Thursby M, Rakela J, McPeak C, Edwards VM, Mosley JW. Studies on the maternal-infant transmission of the viruses which cause acute hepatitis. Gastroenterology. 1981; 80:999–1004.7. Leikin E, Lysikiewicz A, Garry D, Tejani N. Intrauterine transmission of hepatitis A virus. Obstet Gynecol. 1996; 88:690–691.8. McDuffie RS Jr, Bader T. Fetal meconium peritonitis after maternal hepatitis A. Am J Obstet Gynecol. 1999; 180:1031–1032.9. Watson JC, Fleming DW, Borella AJ, Olcott ES, Conrad RE, Baron RC. Vertical transmission of hepatitis A resulting in an outbreak in a neonatal intensive care unit. J Infect Dis. 1993; 167:567–571.10. Tanaka I, Shima M, Kubota Y, Takahashi Y, Kawamata O, Yoshioka A. Vertical transmission of hepatitis A virus. Lancet. 1995; 345:397.11. Erkan T, Kutlu T, Cullu F, Tumay GT. A case of vertical transmission of hepatitis A virus infection. Acta Paediatr. 1998; 87:1008–1009.12. Urganci N, Arapoglu M, Akyildiz B, Nuhoglu A. Neonatal cholestasis resulting from vertical transmission of hepatitis A infection. Pediatr Infect Dis J. 2003; 22:381–382.13. Motte A, Blanc J, Minodier P, Colson P. Acute hepatitis A in a pregnant woman at delivery. Int J Infect Dis. 2009; 13:e49–e51.14. Renge RL, Dani VS, Chitambar SD, Arankalle VA. Vertical transmission of hepatitis A. Indian J Pediatr. 2002; 69:535–536.15. Sohn YM, Rho HO, Park MS, Park JH, Choi BY, Ki M, et al. The changing epidemiology of hepatitis A in children and the consideration of active immunization in Korea. Yonsei Med J. 2000; 41:34–39.16. Willner IR, Uhl MD, Howard SC, Williams EQ, Riely CA, Waters B. Serious hepatitis A: an analysis of patients hospitalized during an urban epidemic in the United States. Ann Intern Med. 1998; 128:111–114.17. Muto Y, Nouri-Aria KT, Meager A, Alexander GJ, Eddleston AL, Williams R. Enhanced tumour necrosis factor and interleukin-1 in fulminant hepatic failure. Lancet. 1988; 2:72–74.18. Torre D, Zeroli C, Giola M, Ferrario G, Fiori GP, Bonetta G, et al. Serum levels of interleukin-1 alpha, interleukin-1 beta, interleukin-6, and tumor necrosis factor in patients with acute viral hepatitis. Clin Infect Dis. 1994; 18:194–198.19. Murtha AP, Greig PC, Jimmerson CE, Herbert WN. Maternal serum interleukin-6 concentration as a marker for impending preterm delivery. Obstet Gynecol. 1998; 91:161–164.20. Romero R, Mazor M, Sepulveda W, Avila C, Copeland D, Williams J. Tumor necrosis factor in preterm and term labor. Am J Obstet Gynecol. 1992; 166:1576–1587.21. Fortunato SJ, Menon RP, Swan KF, Menon R. Inflammatory cytokine (interleukins 1, 6 and 8 and tumor necrosis factor-alpha) release from cultured human fetal membranes in response to endotoxic lipopolysaccharide mirrors amniotic fluid concentrations. Am J Obstet Gynecol. 1996; 174:1855–1861.22. Sadowsky DW, Adams KM, Gravett MG, Witkin SS, Novy MJ. Preterm labor is induced by intraamniotic infusions of interleukin-1beta and tumor necrosis factor-alpha but not by interleukin-6 or interleukin-8 in a nonhuman primate model. Am J Obstet Gynecol. 2006; 195:1578–1589.23. Li XM, Ma L, Yang YB, Shi ZJ, Zhou SS. Clinical characteristics of fulminant hepatitis in pregnancy. World J Gastroenterol. 2005; 11:4600–4603.24. Tan MP, Koren G. Chickenpox in pregnancy: revisited. Reprod Toxicol. 2006; 21:410–420.25. Bell BP, Shapiro CN, Alter MJ, Moyer LA, Judson FN, Mottram K, et al. The diverse patterns of hepatitis A epidemiology in the United States-implications for vaccination strategies. J Infect Dis. 1998; 178:1579–1584.