J Korean Soc Transplant.
2013 Sep;27(3):138-142. 10.4285/jkstn.2013.27.3.138.
Posttransplant Lymphoproliferative Disorder without Epstein-Barr Virus Presented as Small Bowel Perforation in Renal Transplant Recipient: A Case Report
- Affiliations
-
- 1Department of Surgery, Yonsei University College of Medicine, Seoul, Korea. jakii@yuhs.ac
- KMID: 1495872
- DOI: http://doi.org/10.4285/jkstn.2013.27.3.138
Abstract
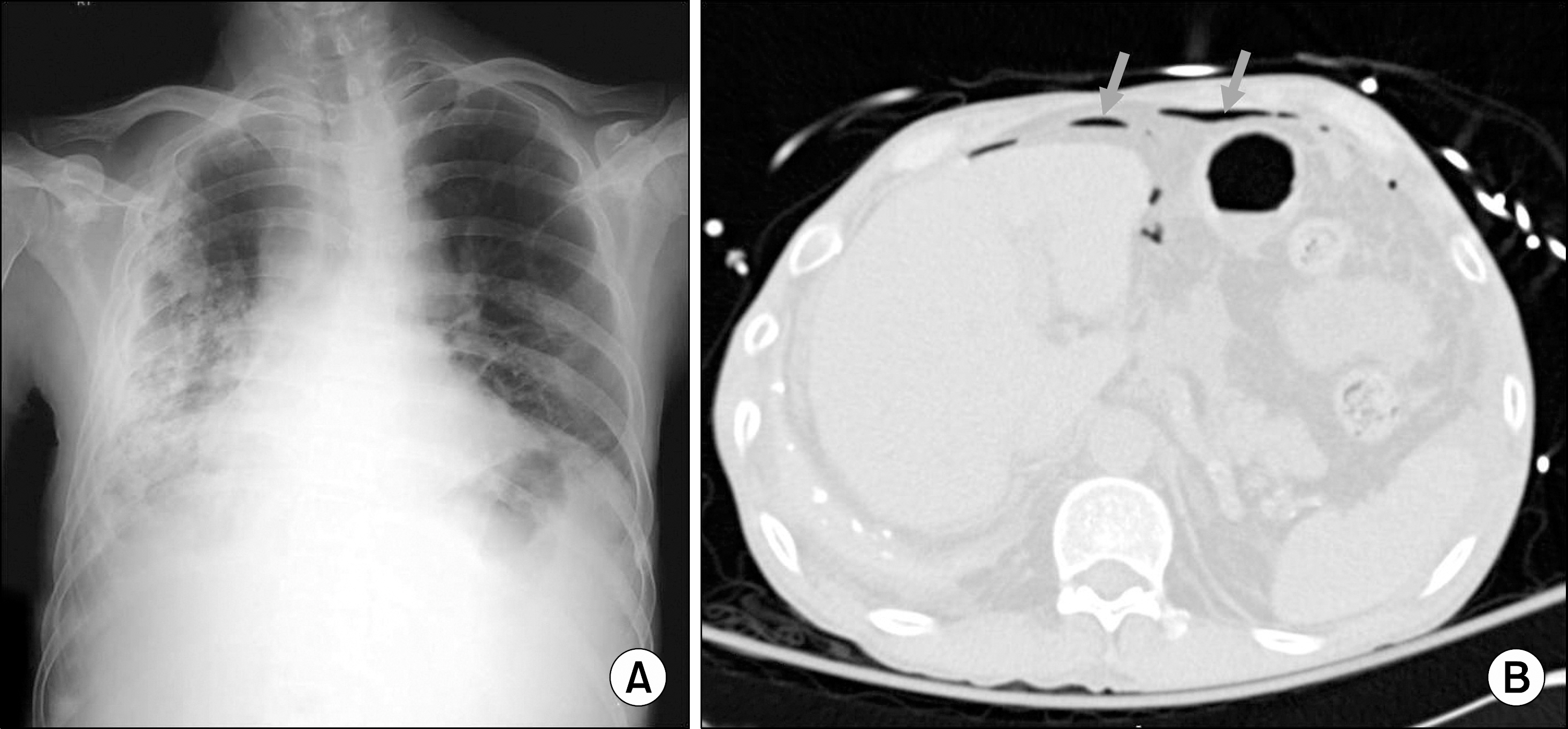
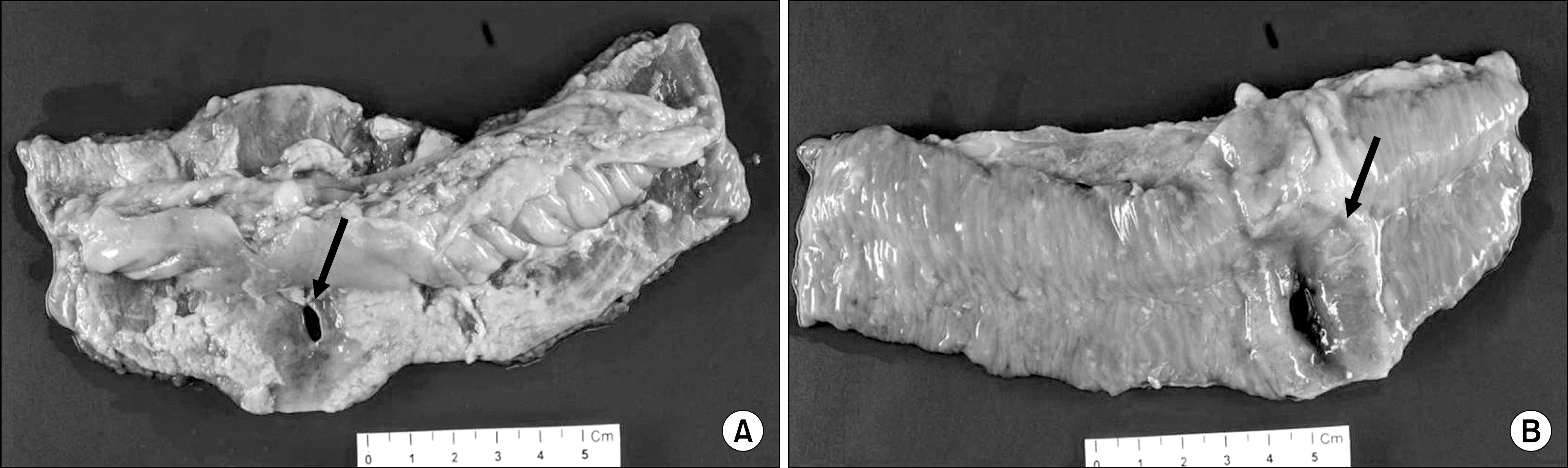
- Posttransplant lymphoproliferative disorder (PTLD) is documented as one of the serious complications leading to mortality particularly in organ transplant recipients receiving immunosuppressive therapy. Extant literature confirms beyond doubt that the most common site of involvement of PTLD is lymph nodes, and rarely involved is the gastrointestinal tract. It is a well-known fact that Epstein-Barr virus (EBV) is a risk factor for PTLD development. In this study, we report a case of PTLD presented as small bowel perforation without EBV infection after long-term immunosuppressive therapy in a renal transplant recipient.
MeSH Terms
Figure
Reference
-
1). Sampaio MS, Cho YW, Qazi Y, Bunnapradist S, Hut-chinson IV, Shah T. Posttransplant malignancies in solid organ adult recipients: an analysis of the U.S. National Transplant Database. Transplantation. 2012; 94:990–8.2). Dharnidharka VR, Tejani AH, Ho PL, Harmon WE. Posttransplant lymphoproliferative disorder in the United States: young Caucasian males are at highest risk. Am J Transplant. 2002; 2:993–8.
Article3). Caillard S, Lamy FX, Quelen C, Dantal J, Lebranchu Y, Lang P, et al. Epidemiology of posttransplant lymphoproliferative disorders in adult kidney and kidney pancreas recipients: report of the French registry and analysis of subgroups of lymphomas. Am J Transplant. 2012; 12:682–93.
Article4). Ferry JA, Jacobson JO, Conti D, Delmonico F, Harris NL. Lymphoproliferative disorders and hematologic malignancies following organ transplantation. Mod Pathol. 1989; 2:583–92.5). Starzl TE, Nalesnik MA, Porter KA, Ho M, Iwatsuki S, Griffith BP, et al. Reversibility of lymphomas and lymphoproliferative lesions developing under cyclosporin- steroid therapy. Lancet. 1984; 1:583–7.6). Buell JF, Gross TG, Woodle ES. Malignancy after transplantation. Transplantation. 2005; 80(2 Suppl):S254–64.
Article7). Cockfield SM. Identifying the patient at risk for post- transplant lymphoproliferative disorder. Transpl Infect Dis. 2001; 3:70–8.8). Shroff R, Rees L. The posttransplant lymphoproliferative disorder: a literature review. Pediatr Nephrol. 2004; 19:369–77.9). Yoon SO, Yu E, Cho YM, Suh C, Kim KM, Han DJ, et al. Posttransplant lymphoproliferative disorders: clin-icopathological analysis of 43 cases in a single center, 1990-2009. Clin Transplant. 2012; 26:67–73.
Article10). Glotz D, Chapman JR, Dharnidharka VR, Hanto DW, Castro MC, Hirsch HH, et al. The Seville expert work-shop for progress in posttransplant lymphoproliferative disorders. Transplantation. 2012; 94:784–93.
Article11). Briggs JD. Causes of death after renal transplantation. Nephrol Dial Transplant. 2001; 16:1545–9.
Article12). Nelson BP, Nalesnik MA, Bahler DW, Locker J, Fung JJ, Swerdlow SH. Epstein-Barr virus-negative posttransplant lymphoproliferative disorders: a distinct entity? Am J Surg Pathol. 2000; 24:375–85.13). Cox KL, Lawrence-Miyasaki LS, Garcia-Kennedy R, Lennette ET, Martinez OM, Krams SM, et al. An increased incidence of Epstein-Barr virus infection and lymphoproliferative disorder in young children on FK506 after liver transplantation. Transplantation. 1995; 59:524–9.14). Cruz RJ Jr, Ramachandra S, Sasatomi E, DiMartini A, de Vera M, Fontes P, et al. Surgical management of gastrointestinal posttransplant lymphoproliferative disorders in liver transplant recipients. Transplantation. 2012; 94:417–23.
Article15). Opelz G, Daniel V, Naujokat C, Fickenscher H, Döhler B. Effect of cytomegalovirus prophylaxis with immunog-lobulin or with antiviral drugs on posttransplant non- Hodgkin lymphoma: a multicentre retrospective analysis. Lancet Oncol. 2007; 8:212–8.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Epstein Barr Virus-Associated Angiocentric T Cell Lymphoma in Nasal Cavity of Renal Transplantation Recipient
- A case of posttransplantation lymphoproliferative disease developed in renal transplant recipient and treated with rituximab
- Epstein-Barr Virus Related Polymorphic Posttransplantation Lymphoproliferative Disease in a Patient with Latent Infection of JC Virus
- Four Cases of Posttransplant Lymphoproliferative Disease (PTLD) Following Renal Transplantation
- Two Cases of Post Transplant Lymphoproliferative Disorder in Lung Transplant Recipients