J Korean Ophthalmol Soc.
2008 Jul;49(7):1087-1093. 10.3341/jkos.2008.49.7.1087.
The Clear Oil-drop Residue After Intravitreal Injection: Comparison between Different Brands of Triamcinolone Acetonide
- Affiliations
-
- 1Department of Ophthalmology, College of Medicine, Hanyang University, Seoul, Korea. Brlee@hanyang.ac.kr
- KMID: 1476962
- DOI: http://doi.org/10.3341/jkos.2008.49.7.1087
Abstract
-
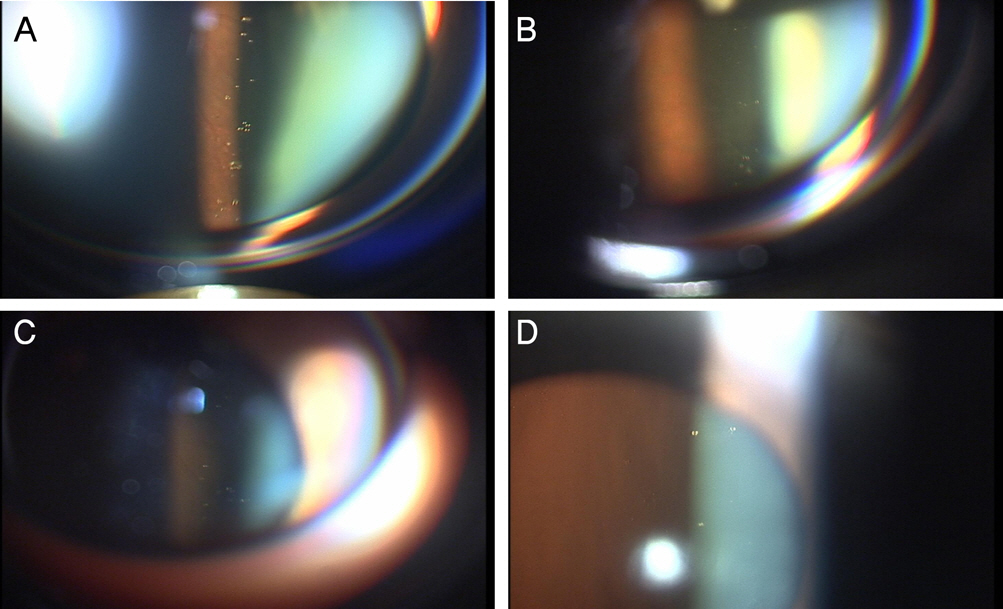
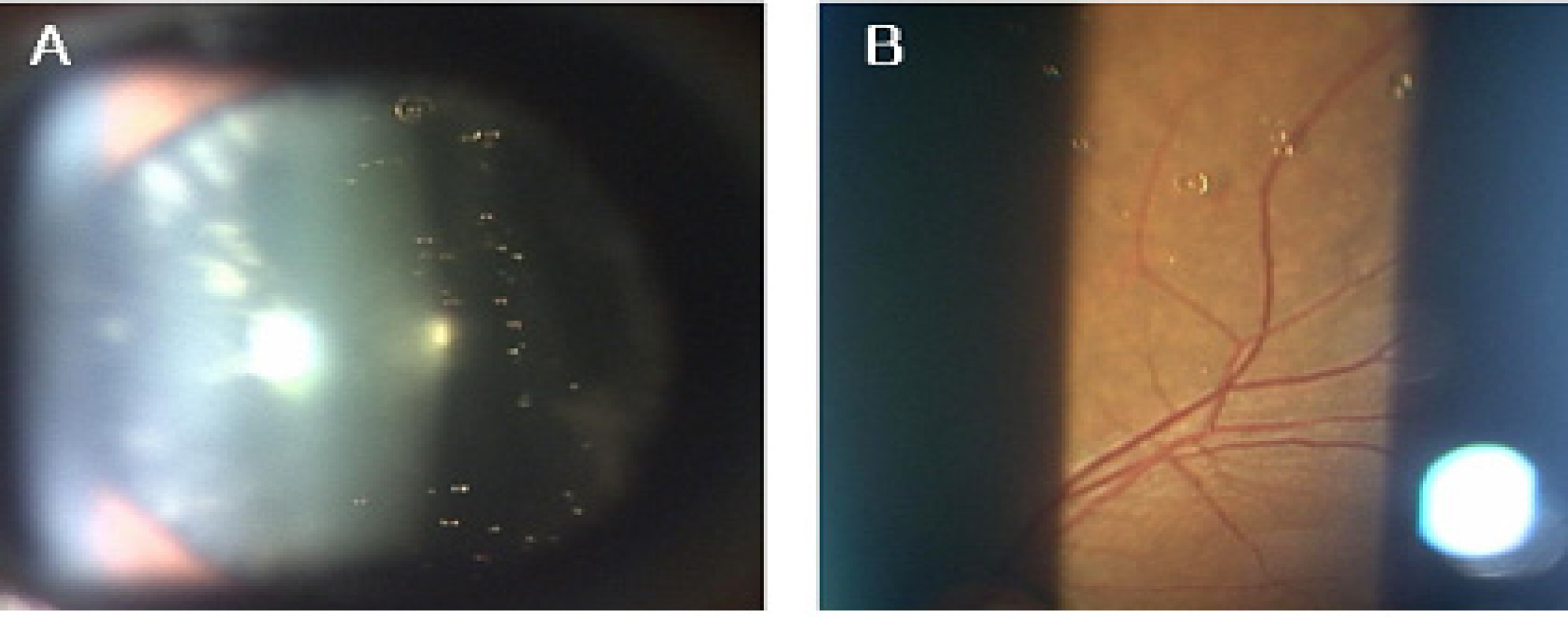
PURPOSE: To investigate the characteristics of the clear oil-drop residue observed in the vitreous cavity after intravitreal injection of different brands of triamcinolone acetonide (TA), and to compare this ingredient's effects in those products.
METHODS
Intravitreal injections of four different brands of triamcinolone acetonide (4 mg/0.1 mL), which are commercially available in Korea, were given to 40 eyes for the treatment of macular edema due to a variety of causes from October 2005 through February 2006. Regular slit-lamp biomicroscopy of fundus had been performed periodically with digital image acquisition equipment after injection of TA for at least two months. We analyzed the characteristics of this residue and compared the number and size of this residue in each product.
RESULTS
Four TA products commercially available in Korea were used, with vehicles composed of preservatives and suspending agents. There were differences between products in respect to ingredients and content, as well as the characteristics of the intravitreal clear oil droplet-like residue.
CONCLUSIONS
Each TA product has a variable frequency and variable amounts of clear oil droplet-like residue, which seems to be a component of the vehicle. This variation could generate differences in efficacy, side effects, and retaining duration. It should be verified whether commercial TA products are consistently safe and effective.
MeSH Terms
Figure
Cited by 1 articles
-
Treatment of Diabetic Macular Edema: A Comparative Study
Yong Jun Lee, Kyung Seek Choi, Sung Jin Lee
J Korean Ophthalmol Soc. 2010;51(6):849-859. doi: 10.3341/jkos.2010.51.6.849.
Reference
-
References
1. Kai W, Yanrong J, Xiaoxin L. Vehicle of triamcinolone acetonide is associated with retinal toxicity and transient lens density. Graefes Arch Clin Exp Ophthalmol. 2006; 244:1152–9.2. Narayanan R, Mungcal JK, Kenney MC. . Toxicity of triamcinolone acetonide on retinal neurosensory and pigment epithelial cells. Invest Ophthalmol Vis Sci. 2006; 47:722–8.
Article3. Morrison VL, Koh HJ, Cheng L. . Intravitreal toxicity of the kenalog vehicle (benzyl alcohol) in rebbits. Retina. 2006; 26:339–44.4. Kang SM, Park YS, Lee BR. The Clear Oil-drop Residue in the Vitreous Cavity after Intravitreal Triamcinolone Injection. J Korean Ophthalmol Soc. 2007; 48:665–70.5. Yu SY, Damico FM, Viola F. . Retinal toxicity of intravitreal triamcinolone acetonide: A morphological study. Retina. 2006; 26:531–6.6. Yeung CK, Chan KP, Chan CK. . Cytotoxicity of triamcinolone on cultured human retinal pigment epithelial cells: comparison with dexamethasone and hydrocortisone. Jpn J Ophthalmol. 2004; 48:236–42.
Article7. Morrison VL, Koh HJ, Cheng L. . Intravitreal toxicity of the kenalog vehicle (benzyl alcohol) in rabbits. Retina. 2006; 26:339–44.
Article8. Kai W, Yanrong J, Xiaoxin L. Vehicle of triamcinolone acetonide is associated with retinal toxicity and transient increase of lens density. Greafes Arch Clin Exp Ophthalmol. 2006; 2:1–8.
Article9. Narayan R, Mungcal JK, Kenney MC. . Toxicity of triamcinolone acetonide on retinal neurosensory and pigment epithelial cells. Invest Ophthalmol Vis Sci. 2006; 47:722–8.10. Yeung CK, Chan KP, Chiang SWY. . The toxic and stress responses of cultured human retinal pigment epithelium (ARPE19) and human glial cells (SVG) in the presence of triamcinolone. Invest Ophthalmol Vis Sci. 2003; 44:5293–300.
Article11. Beaudouin E, Kanny G, Gueant JL. Anaphylaxis caused by carboxymethylcellulose: report of 2 cases of shock from injectable corticoids. Allerg Immunol. 1992; 24:333–5.12. Yoshikawa K, Kotake S, Ichiishi A. . Posterior sub-Tenon injections if repository corticosteroids in uveitis patients with cystoid macular edema. Jpn J Ophthalmol. 1995; 39:71–6.13. McGhee CN, Dean S, Danesh-Meyer H. Locally administered ocular corticosteroids: benefits and risks. Drug Saf. 2002; 25:33–55.14. Hernaez-Ortega MC, Soto-Pedre E.A simple and rapid method for purification of triamcinolone acetonide suspension for intravitreal injection. Ophthalmic Surg Lasers Imaging. 2004; 35:350–1.
Article15. Nishimura A, Kovayashi A, Segawa Y. . Isolating triamacinolone acetonide particles for intravitreal use with a porous membrane filter. Retina. 2003; 23:777–9.16. Walker TD. Benzalkonium toxicity. Clin Experiment Ophthalmol. 2004; 32:657.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Clear Oil-drop Residue in the Vitreous Cavity after Intravitreal Triamcinolone Injection
- Intravitreal and Additional Posterior Subtenon Triamcinolone Injection in Diabetic Macular Edema
- Change of Residual Period and Clearance Rate of Intravitreal Triamcinolone According to Initial Injection Dosage
- The Effect of Intravitreal Triamcinolone Acetonide on Intraocular Pressure
- Intravitreal Injection of Triamcinolone Acetonide in Vitrectomy with Silicone Oil Placement