Tuberc Respir Dis.
2010 Jun;68(6):328-333. 10.4046/trd.2010.68.6.328.
Interferon-gamma Release Assay among Tuberculin Skin Test Positive Students in Korean High Schools
- Affiliations
-
- 1Department of Microbiology, Korean Institute of Tuberculosis, Seoul, Korea. ypark7@empal.com
- 2Division of HIV and Tuberculosis Control, Center for Disease Control, Korea Centers for Disease Control & Prevention, Seoul, Korea.
- 3Division of Pulmonary and Critical Care Medicine, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- KMID: 1458041
- DOI: http://doi.org/10.4046/trd.2010.68.6.328
Abstract
- BACKGROUND
There are several active tuberculosis (TB) cases in Korean high schools each school year. The risk of transmission in schools is extremely high due to the considerable time spent in closed classrooms. We evaluated the control of latent tuberculosis infection in Korean high schools.
METHODS
When a student was identified with active TB, tuberculin skin testing was performed on their classmates and on students in their same school grade. When a student had a positive tuberculin skin tests (TST), they underwent follow-up testing with QuantiFERON-TB Gold In-Tube (QFT). The manufacturer recommended a cut-off of 0.35 IU/mL to determine QFT positivity was applied.
RESULTS
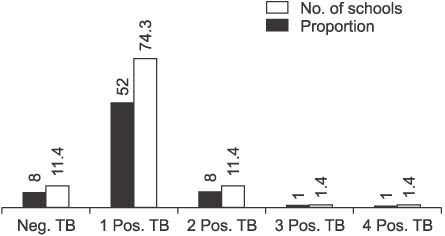
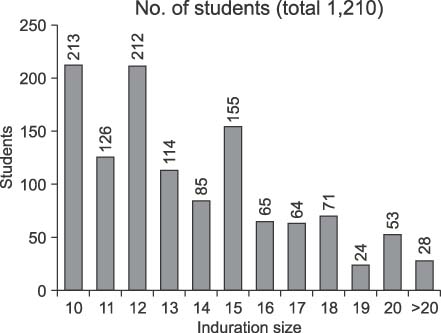
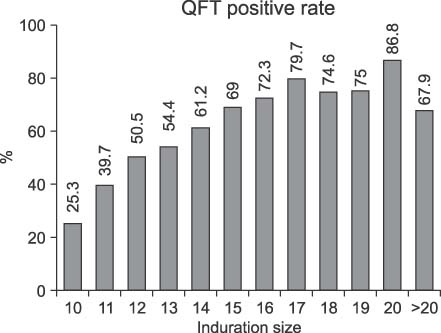
A total of 131 pulmonary tuberculosis (TB) patients were included based on the criteria for screening TB contacts in the National Tuberculosis Control Program. Seventy-five (57.2%) students tested smear positive. TST were performed on 7,109 students who were classmates of, or in the same grade as, a TB patient. Of the contacts, 1,231 students (17.3%) were TST positive and they were screened with QFT. Six hundred-sixty-six (55.0%) of the tested students returned a positive QFT result and the rate of positivity was significantly associated with the increasing size of TST indurations (p<0.0001).
CONCLUSION
The use of QFT resulted in approximately 45% of TST positive students not being given chemoprophylaxis.
MeSH Terms
Figure
Reference
-
1. World Health Organization. Global tuberculosis control: epidemiology, strategy, financing: WHO report 2009. 2009. Geneva: World Health Organization.2. Korea Centers for Disease Control and Prevention. Annual report on the notified tuberculosis patients in Korea. 2007. Seoul: Korea Centers for Disease Control and Prevention.3. Korea Centers for Disease Control and Prevention. Guidelines of the national tuberculosis control program. 2008. Seoul: Korea Centers Disease Control and Prevention.4. Kang YA, Lee HW, Yoon HI, Cho B, Han SK, Shim YS, et al. Discrepancy between the tuberculin skin test and the whole-blood interferon gamma assay for the diagnosis of latent tuberculosis infection in an intermediate tuberculosis-burden country. JAMA. 2005. 293:2756–2761.5. Choi CM, Kang CI, Kim DH, Kim CH, Kim HJ, Lee CH, et al. The role of TST in the diagnosis of latent tuberculosis infection among military personnel in South Korea. Int J Tuberc Lung Dis. 2006. 10:1342–1346.6. Pai M, Gokhale K, Joshi R, Dogra S, Kalantri S, Mendiratta DK, et al. Mycobacterium tuberculosis infection in health care workers in rural India: comparison of a whole-blood interferon gamma assay with tuberculin skin testing. JAMA. 2005. 293:2746–2755.7. Nienhaus A, Schablon A, Diel R. Interferon-gamma release assay for the diagnosis of latent TB infection: analysis of discordant results, when compared to the tuberculin skin test. PLoS One. 2008. 3:e2665.8. Vinton P, Mihrshahi S, Johnson P, Jenkin GA, Jolley D, Biggs BA. Comparison of QuantiFERON-TB Gold In-Tube Test and tuberculin skin test for identification of latent Mycobacterium tuberculosis infection in healthcare staff and association between positive test results and known risk factors for infection. Infect Control Hosp Epidemiol. 2009. 30:215–221.9. Harada N, Higuchi K, Yoshiyama T, Kawabe Y, Fujita A, Sasaki Y, et al. Comparison of the sensitivity and specificity of two whole blood interferon-gamma assays for M. tuberculosis infection. J Infect. 2008. 56:348–353.10. Bugiani M, Borraccino A, Migliore E, Carosso A, Piccioni P, Cavallero M, et al. Tuberculin reactivity in adult BCG-vaccinated subjects: a cross-sectional study. Int J Tuberc Lung Dis. 2003. 7:320–326.11. Mauch H, Brehmer W. Mycobacterial antibodies after tuberculin testing, BCG- vaccination, BCG-immunotherapy and against cross-reacting antigens in a solid-phase radioimmunoassay. Zentralbl Bakteriol Mikrobiol Hyg A. 1982. 251:380–388.12. Hong YP, Kim SJ, Lew WJ, Lee EK, Han YC. The seventh nationwide tuberculosis prevalence survey in Korea, 1995. Int J Tuberc Lung Dis. 1998. 2:27–36.13. Diel R, Wrighton-Smith P, Zellweger JP. Cost-effectiveness of interferon-gamma release assay testing for the treatment of latent tuberculosis. Eur Respir J. 2007. 30:321–332.14. Chun JK, Kim CK, Kim HS, Jung GY, Lee TJ, Kim KH, et al. The role of a whole blood interferon-gamma assay for the detection of latent tuberculosis infection in Bacille Calmette-Guerin vaccinated children. Diagn Microbiol Infect Dis. 2008. 62:389–394.15. Eum SY, Lee YJ, Kwak HK, Min JH, Hwang SH, Via LE, et al. Evaluation of the diagnostic utility of a whole-blood interferon-gamma assay for determining the risk of exposure to Mycobacterium tuberculosis in Bacille Calmette-Guerin (BCG)-vaccinated individuals. Diagn Microbiol Infect Dis. 2008. 61:181–186.16. Higuchi K, Harada N, Mori T, Sekiya Y. Use of QuantiFERON-TB Gold to investigate tuberculosis contacts in a high school. Respirology. 2007. 12:88–92.17. Harada N, Higuchi K, Sekiya Y, Rothel J, Kitoh T, Mori T. Basic characteristics of a novel diagnostic method (QuantiFERON TB-2G) for latent tuberculosis infection with the use of Mycobacterium tuberculosis-specific antigens, ESAT-6 and CFP-10. Kekkaku. 2004. 79:725–735.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Proposal to Revise the Screening Test for Latent Tuberculosis Infection in Close Contacts at Elementary Schools in Korea
- An Usefulness of In Vitro Interferon Gamma Assay for the Diagnosis of Latent Tuberculosis Infection in Middle- and High-School Students in Jeju-Shi, Korea
- Diagnosis and treatment of latent tuberculosis infection
- Comparison of Interferon-gamma Assays with the Tuberculin Skin Test in Children
- Accuracy of an Interferon-gamma Release Assay to Detect Active Tuberculosis in Children: A Pilot Study