Immune Netw.
2011 Aug;11(4):203-209. 10.4110/in.2011.11.4.203.
Identification of CCL1 as a Gene Differentially Expressed in CD4+ T Cells Expressing TIM-3
- Affiliations
-
- 1Department of Microbiology, Ajou University School of Medicine, Suwon 442-749, Korea. sinsun@ajou.ac.kr
- 2Graduate Program of Molecular Medicine, Ajou University School of Medicine, Suwon 442-749, Korea.
- KMID: 1449811
- DOI: http://doi.org/10.4110/in.2011.11.4.203
Abstract
- BACKGROUND
T cell immunoglobulin mucin containing molecule (TIM)-3 is expressed in differentiated Th1 cells and is involved in the suppression of the cytokine production by these cells. However, the regulation of the expression of other T cell genes by TIM-3 is unclear. Herein, we attempted to identify differentially expressed genes in cells abundantly expressing TIM-3 compared to cells with low expression of TIM-3.
METHODS
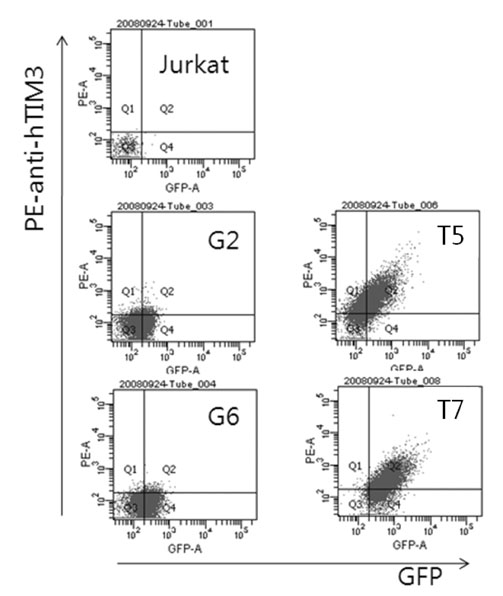
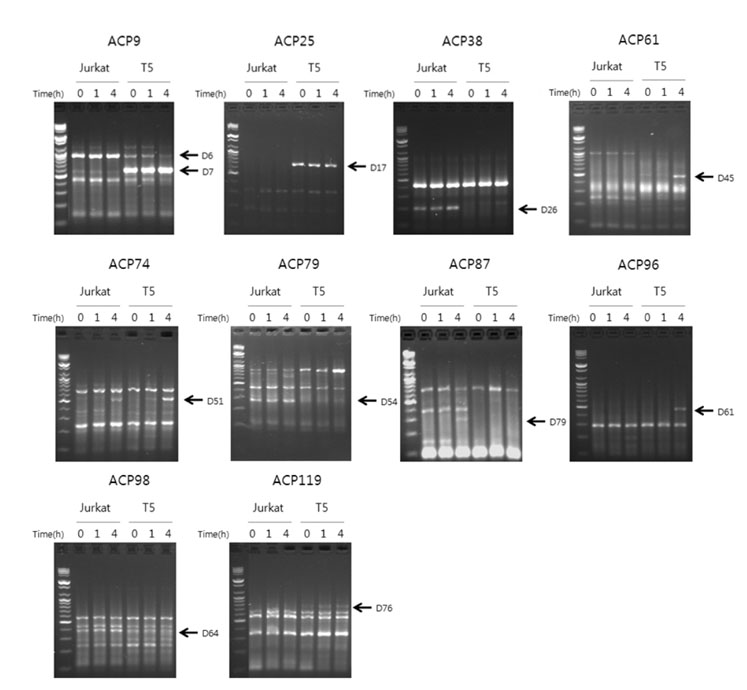
TIM-3 overexpressing cell clones were established by transfection of Jurkat T cells with TIM-3 expression vector. For screening of differentially expressed genes, gene fishing technology based on reverse transcription-polymerase chain reaction (RT-PCR) using an annealing control primer system was used. The selected candidate genes were validated by semi quantitative and real-time RT-PCR.
RESULTS
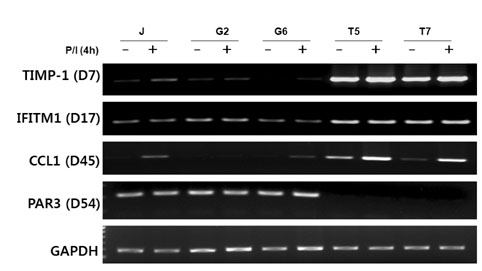
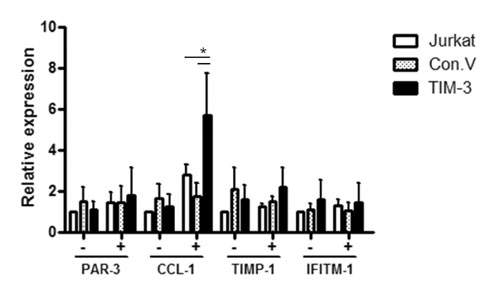
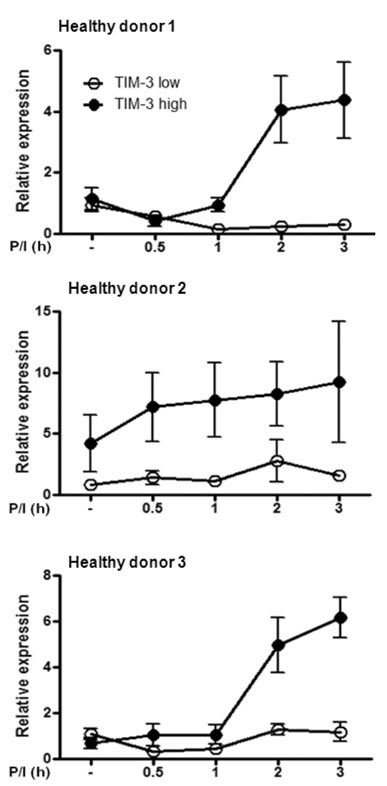
The transcription of TIMP-1, IFITM1, PAR3 and CCL1 was different between TIM-3 overexpressing cells and control cells. However, only CCL1 transcription was significantly different in cells transiently transfected with TIM3 expression vector compared with control cells. CCL1 transcription was increased in primary human CD4+ T cells abundantly expressing TIM-3 but not in cells with low expression of TIM-3.
CONCLUSION
CCL1 was identified as a differentially transcribed gene in TIM-3-expressing CD4+ T cells.
Keyword
MeSH Terms
Figure
Reference
-
1. Anderson AC, Anderson DE. TIM-3 in autoimmunity. Curr Opin Immunol. 2006. 18:665–669.
Article2. Sabatos CA, Chakravarti S, Cha E, Schubart A, Sánchez-Fueyo A, Zheng XX, Coyle AJ, Strom TB, Freeman GJ, Kuchroo VK. Interaction of Tim-3 and Tim-3 ligand regulates T helper type 1 responses and induction of peripheral tolerance. Nat Immunol. 2003. 4:1102–1110.
Article3. Lee MJ, Woo MY, Heo YM, Kim JS, Kwon MH, Kim K, Park S. The inhibition of the T-cell immunoglobulin and mucin domain 3 (Tim3) pathway enhances the efficacy of tumor vaccine. Biochem Biophys Res Commun. 2010. 402:88–93.
Article4. Monney L, Sabatos CA, Gaglia JL, Ryu A, Waldner H, Chernova T, Manning S, Greenfield EA, Coyle AJ, Sobel RA, Freeman GJ, Kuchroo VK. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature. 2002. 415:536–541.
Article5. Sánchez-Fueyo A, Tian J, Picarella D, Domenig C, Zheng XX, Sabatos CA, Manlongat N, Bender O, Kamradt T, Kuchroo VK, Gutiérrez-Ramos JC, Coyle AJ, Strom TB. Tim-3 inhibits T helper type 1-mediated auto- and alloimmune responses and promotes immunological tolerance. Nat Immunol. 2003. 4:1093–1101.
Article6. Liu Y, Shu Q, Gao L, Hou N, Zhao D, Liu X, Zhang X, Xu L, Yue X, Zhu F, Guo C, Liang X, Ma C. Increased Tim-3 expression on peripheral lymphocytes from patients with rheumatoid arthritis negatively correlates with disease activity. Clin Immunol. 2010. 137:288–295.
Article7. Yang L, Anderson DE, Kuchroo J, Hafler DA. Lack of TIM-3 immunoregulation in multiple sclerosis. J Immunol. 2008. 180:4409–4414.
Article8. Morimoto K, Hosomi S, Yamagami H, Watanabe K, Kamata N, Sogawa M, Machida H, Okazaki H, Tanigawa T, Nagahara H, Noda E, Tominaga K, Watanabe T, Fujiwara Y, Maeda K, Hirakawa K, Arakawa T. Dysregulated upregulation of T-cell immunoglobulin and mucin domain-3 on mucosal T helper 1 cells in patients with Crohn's disease. Scand J Gastroenterol. 2011. 46:701–709.
Article9. Matsumoto R, Matsumoto H, Seki M, Hata M, Asano Y, Kanegasaki S, Stevens RL, Hirashima M. Human ecalectin, a variant of human galectin-9, is a novel eosinophil chemoattractant produced by T lymphocytes. J Biol Chem. 1998. 273:16976–16984.
Article10. Zhu C, Anderson AC, Schubart A, Xiong H, Imitola J, Khoury SJ, Zheng XX, Strom TB, Kuchroo VK. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol. 2005. 6:1245–1252.
Article11. DeKruyff RH, Bu X, Ballesteros A, Santiago C, Chim YL, Lee HH, Karisola P, Pichavant M, Kaplan GG, Umetsu DT, Freeman GJ, Casasnovas JM. T cell/transmembrane, Ig, and mucin-3 allelic variants differentially recognize phosphatidylserine and mediate phagocytosis of apoptotic cells. J Immunol. 2010. 184:1918–1930.
Article12. Iellem A, Colantonio L, Bhakta S, Sozzani S, Mantovani A, Sinigaglia F, D'Ambrosio D. Inhibition by IL-12 and IFN-alpha of I-309 and macrophage-derived chemokine production upon TCR triggering of human Th1 cells. Eur J Immunol. 2000. 30:1030–1039.
Article13. Su SB, Grajewski RS, Luger D, Agarwal RK, Silver PB, Tang J, Tuo J, Chan CC, Caspi RR. Altered chemokine profile associated with exacerbated autoimmune pathology under conditions of genetic interferon-gamma deficiency. Invest Ophthalmol Vis Sci. 2007. 48:4616–4625.
Article14. Selvan RS, Zhou LJ, Krangel MS. Regulation of I-309 gene expression in human monocytes by endogenous interleukin-1. Eur J Immunol. 1997. 27:687–694.
Article15. Gonzalo JA, Qiu Y, Lora JM, Al-Garawi A, Villeval JL, Boyce JA, Martinez-A C, Marquez G, Goya I, Hamid Q, Fraser CC, Picarella D, Cote-Sierra J, Hodge MR, Gutierrez-Ramos JC, Kolbeck R, Coyle AJ. Coordinated involvement of mast cells and T cells in allergic mucosal inflammation: critical role of the CC chemokine ligand 1:CCR8 axis. J Immunol. 2007. 179:1740–1750.
Article16. Zingoni A, Soto H, Hedrick JA, Stoppacciaro A, Storlazzi CT, Sinigaglia F, D'Ambrosio D, O'Garra A, Robinson D, Rocchi M, Santoni A, Zlotnik A, Napolitano M. The chemokine receptor CCR8 is preferentially expressed in Th2 but not Th1 cells. J Immunol. 1998. 161:547–551.17. Sebastiani S, Allavena P, Albanesi C, Nasorri F, Bianchi G, Traidl C, Sozzani S, Girolomoni G, Cavani A. Chemokine receptor expression and function in CD4+ T lymphocytes with regulatory activity. J Immunol. 2001. 166:996–1002.
Article18. Montes-Vizuet R, Vega-Miranda A, Valencia-Maqueda E, Negrete-García MC, Velásquez JR, Teran LM. CC chemokine ligand 1 is released into the airways of atopic asthmatics. Eur Respir J. 2006. 28:59–67.
Article19. Gombert M, Dieu-Nosjean MC, Winterberg F, Bünemann E, Kubitza RC, Da Cunha L, Haahtela A, Lehtimäki S, Müller A, Rieker J, Meller S, Pivarcsi A, Koreck A, Fridman WH, Zentgraf HW, Pavenstädt H, Amara A, Caux C, Kemeny L, Alenius H, Lauerma A, Ruzicka T, Zlotnik A, Homey B. CCL1-CCR8 interactions: an axis mediating the recruitment of T cells and Langerhans-type dendritic cells to sites of atopic skin inflammation. J Immunol. 2005. 174:5082–5091.
Article20. Soler D, Chapman TR, Poisson LR, Wang L, Cote-Sierra J, Ryan M, McDonald A, Badola S, Fedyk E, Coyle AJ, Hodge MR, Kolbeck R. CCR8 expression identifies CD4 memory T cells enriched for FOXP3+ regulatory and Th2 effector lymphocytes. J Immunol. 2006. 177:6940–6951.
Article21. Iellem A, Mariani M, Lang R, Recalde H, Panina-Bordignon P, Sinigaglia F, D'Ambrosio D. Unique chemotactic response profile and specific expression of chemokine receptors CCR4 and CCR8 by CD4(+)CD25(+) regulatory T cells. J Exp Med. 2001. 194:847–853.
Article22. Hoshino A, Kawamura YI, Yasuhara M, Toyama-Sorimachi N, Yamamoto K, Matsukawa A, Lira SA, Dohi T. Inhibition of CCL1-CCR8 interaction prevents aggregation of macrophages and development of peritoneal adhesions. J Immunol. 2007. 178:5296–5304.
Article23. Hastings WD, Anderson DE, Kassam N, Koguchi K, Greenfield EA, Kent SC, Zheng XX, Strom TB, Hafler DA, Kuchroo VK. TIM-3 is expressed on activated human CD4+ T cells and regulates Th1 and Th17 cytokines. Eur J Immunol. 2009. 39:2492–2501.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Binding Properties of Glycosylated and Non-Glycosylated Tim-3 Molecules on CD4+CD25+ T Cells
- CCL1, Specific Manifestation in Lesional Atopic Skin
- Molecular variations in Th1-specific cell surface gene Tim-3
- Identification of Differentially Expressed Genes in Kawasaki Disease Using Annealing Control Primer System
- Identification of Differentially Expressed Radiation-induced Genes in Cervix Carcinoma Cells Using Suppression Subtractive Hybridization