Hip Pelvis.
2012 Jun;24(2):133-138. 10.5371/hp.2012.24.2.133.
Effect of Intravenous Administration of Bisphosphonate for Patients Operatively Treated for Osteoporotic Hip Fracture
- Affiliations
-
- 1Department of Orthopaedic Surgery, College of Medicine, Chosun University, Gwangju, Korea. shalee@chosun.ac.kr
- KMID: 1439102
- DOI: http://doi.org/10.5371/hp.2012.24.2.133
Abstract
- PURPOSE
We evaluated changes in bone mineral density and biochemical bone turn over markers resulting from intravenous administration of zoledronic acid for the purpose of increasing bone mineral density and decreasing bone turnover rate in patients who had received operative treatment after hip fracture.
MATERIALS AND METHODS
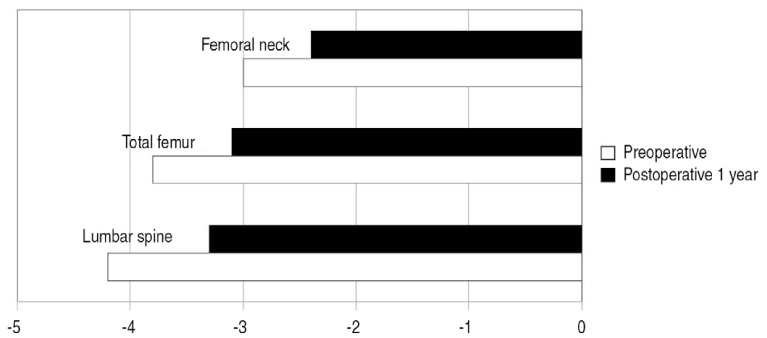
We carried out a retrospective study of 34 patients who had received injections of zoledronic acid after surgical treatment for hip fracture from January 2009 to June 2010, with a follow up period of more than one year. We evaluated pre and post T-scores of DXA in spine, proximal femur and femoral neck along with biochemical bone metabolic markers, and we then analyzed each factor.
RESULTS
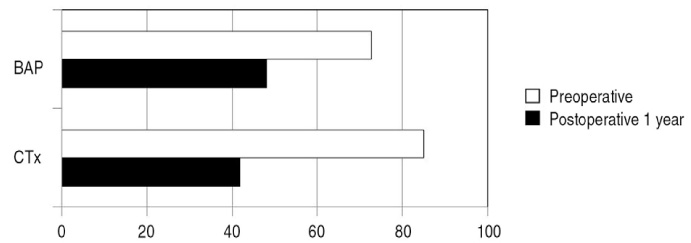
T score was enhanced in all cases with pre T-score -4.2 and post T-score -3.3 revealing statistical significance (P<0.05). In addition, two biochemical bone turnover markers were observed to decrease in most patients. Three days after drug administration, 7 patients(20.6%) had minor adverse effects. There were no serious complications such as atrial fibrillation.
CONCLUSION
No major adverse effects were observed, only minor ones in patients who had been injected with zoledronic acid for the prevention of osteoporotic fracture after surgical treatment for hip fracture. We confirmed the affirmative effects on changes in bone mineral density and biochemical bone turn over markers associated with the use of this drug.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
A Interventional Study in a Real Life Setting to Assess the Clinical Efficacy and Effect on Fracture in the 1 Year after Injection of Zoledronic Acid in Osteoporotic Patients with Long Bone or Spine, Pelvic Fractures
Jaewon Lee, Joonguk Kim, Byeungjik Kang, Jaedong Kim, Ki-Chul Park, Ye-Soo Park
J Korean Orthop Assoc. 2016;51(4):320-326. doi: 10.4055/jkoa.2016.51.4.320.
Reference
-
1. Boonen S, Autier P, Barette M, Vanderschueren D, Lips P, Haentjens P. Functional outcome and quality of life following hip fracture in elderly women: a prospective controlled study. Osteoporos Int. 2004. 15:87–94.
Article2. Davidson CW, Merrilees MJ, Wilkinson TJ, McKie JS, Gilchrist NL. Hip fracture mortality and morbidity--can we do better? N Z Med J. 2001. 114:329–332.3. Lee SR, Kim SR, Chung KH, et al. Mortality and activity after hip fracture: a prospective study. J Korean Orthop Assoc. 2005. 40:423–427.
Article4. Magaziner J, Lydick E, Hawkes W, et al. Excess mortality attributable to hip fracture in white women aged 70 years and older. Am J Public Health. 1997. 87:1630–1636.
Article5. Moon ES, Kim HS, Park JO, et al. The incidence of new vertebral compression fractures in women after kyphoplasty and factors involved. Yonsei Med J. 2007. 48:645–652.
Article6. Yoon HK, Cho DY, Shin DE, Song SJ, Kim JH, Yoon BH. Clinical distribution of bilateral non-contemporary hip fractures in elderly patients. J Korean Fract Soc. 2005. 18:375–378.
Article7. Gardner MJ, Flik KR, Mooar P, Lane JM. Improvement in the undertreatment of osteoporosis following hip fracture. J Bone Joint Surg Am. 2002. 84-A:1342–1348.
Article8. Ha YC, Kim SR, Koo KH, et al. An epidemiological study of hip fracture in Jeju Island, Korea. J Korean Orthop Assoc. 2004. 39:131–136.
Article9. Lyles KW, Colón-Emeric CS, Magaziner JS, et al. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med. 2007. 357:1799–1809.
Article10. Black DM, Cummings SR, Karpf DB, et al. Fracture Intervention Trial Research Group. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Lancet. 1996. 348:1535–1541.
Article11. Miller PD, McClung MR, Macovei L, et al. Monthly oral ibandronate therapy in postmenopausal osteoporosis: 1-year results from the MOBILE study. J Bone Miner Res. 2005. 20:1315–1322.
Article12. Reginster JY, Adami S, Lakatos P, et al. Efficacy and tolerability of once-monthly oral ibandronate in postmenopausal osteoporosis: 2 year results from the MOBILE study. Ann Rheum Dis. 2006. 65:654–661.
Article13. Cramer JA, Amonkar MM, Hebborn A, Altman R. Compliance and persistence with bisphosphonate dosing regimens among women with postmenopausal osteoporosis. Curr Med Res Opin. 2005. 21:1453–1460.
Article14. Downey TW, Foltz SH, Boccuzzi SJ, Omar MA, Kahler KH. Adherence and persistence associated with the pharmacologic treatment of osteoporosis in a managed care setting. South Med J. 2006. 99:570–575.
Article15. Cooper A, Drake J, Brankin E. PERSIST Investigators. Treatment persistence with once-monthly ibandronate and patient support vs. once-weekly alendronate: results from the PERSIST study. Int J Clin Pract. 2006. 60:896–905.
Article16. Black DM, Delmas PD, Eastell R, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007. 356:1809–1822.
Article17. Chesnut CH 3rd, Bell NH, Clark GS, et al. Hormone replacement therapy in postmenopausal women: urinary N-telopeptide of type I collagen monitors therapeutic effect and predicts response of bone mineral density. Am J Med. 1997. 102:29–37.
Article18. el-Hajj Fuleihan G, Brown EM, Curtis K, et al. Effect of sequential and daily continuous hormone replacement therapy on indexes of mineral metabolism. Arch Intern Med. 1992. 152:1904–1909.
Article19. Rosen LS, Gordon D, Tchekmedyian NS, et al. Long-term efficacy and safety of zoledronic acid in the treatment of skeletal metastases in patients with nonsmall cell lung carcinoma and other solid tumors: a randomized, Phase III, double-blind, placebo-controlled trial. Cancer. 2004. 100:2613–2621.
Article20. Bobyn JD, Hacking SA, Krygier JJ, Harvey EJ, Little DG, Tanzer M. Zoledronic acid causes enhancement of bone growth into porous implants. J Bone Joint Surg Br. 2005. 87:416–420.
Article21. Bragdon CR, Doherty AM, Jasty M, Rubash H, Harris WH. Effect of oral alendronate on net bone ingrowth into canine cementless total hips. J Arthroplasty. 2005. 20:258–263.
Article22. Zou X, Xue Q, Li H, Bünger M, Lind M, Bünge C. Effect of alendronate on bone ingrowth into porous tantalum and carbon fiber interbody devices: an experimental study on spinal fusion in pigs. Acta Orthop Scand. 2003. 74:596–603.
Article23. Lee YK, Kim KC, Choi HY, Jang J, Koo KH, Ha YC. Intravenous zolendronic acid for the patients treated operatively after hip fracture: a short-term safety prospective cohort study. J Korean Hip Soc. 2008. 20:305–310.
Article24. Diel IJ, Bergner R, Grotz KA. Adverse effects of bisphosphonates: current issues. J Support Oncol. 2007. 5:475–482.25. Novince CM, Ward BB, McCauley LK. Osteonecrosis of the jaw: an update and review of recommendations. Cells Tissues Organs. 2009. 189:275–283.
Article26. Sarin J, DeRossi SS, Akintoye SO. Updates on bisphosphonates and potential pathobiology of bisphosphonate-induced jaw osteonecrosis. Oral Dis. 2008. 14:277–285.
Article27. Allen MR, Reinwald S, Burr DB. Alendronate reduces bone toughness of ribs without significantly increasing microdamage accumulation in dogs following 3 years of daily treatment. Calcif Tissue Int. 2008. 82:354–360.
Article28. Goh SK, Yang KY, Koh JS, et al. Subtrochanteric insufficiency fractures in patients on alendronate therapy: a caution. J Bone Joint Surg Br. 2007. 89:349–353.29. Neviaser AS, Lane JM, Lenart BA, Edobor-Osula F, Lorich DG. Low-energy femoral shaft fractures associated with alendronate use. J Orthop Trauma. 2008. 22:346–350.
Article30. Black DM, Reid IR, Boonen S, et al. The effect of 3 versus 6 years of zoledronic acid treatment of osteoporosis: a randomized extension to the HORIZON-Pivotal Fracture Trial (PFT). J Bone Miner Res. 2012. 27:243–254.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Intravenous Zolendronic Acid for the Patients Treated Operatively after Hip Fracture: A Short-Term Safety Prospective Cohort Study
- Influence of Oral and Intravenous Bisphosphonate for the Patients Treated Surgically in Osteoporotic Distal Radius Fracture
- Does the Time of Postoperative Bisphosphonate Administration Affect the Bone Union in Osteoporotic Intertrochanteric Fracture of Femur?
- Analysis of the Adherence in Once Yearly Intravenous Zoledronic Acid
- Influence of Early Bisphosphonate Administration for Fracture Healing in Patients with Osteoporotic Proximal Humerus Fractures