J Korean Med Assoc.
2012 Aug;55(8):729-740. 10.5124/jkma.2012.55.8.729.
Establishing semantic interoperability in the course of clinical document exchange using international standard for metadata registry
- Affiliations
-
- 1Seoul National University Biomedical Informatics (SNUBI), Division of Biomedical Informatics, Seoul National University College of Medicine, Seoul, Korea. juhan@snu.ac.kr
- 2Department of Laboratory Medicine, Pusan National University School of Medicine, Busan, Korea.
- 3Medical Research Institute, Pusan National University Hospital, Busan, Korea.
- 4Heart Center of Chonnam National University Hospital, Gwangju, Korea.
- 5Department of Biomedical Informatics, Ajou University School of Medicine, Suwon, Korea.
- 6U-Healthcare Center, Gachon University Gil Hospital, Incheon, Korea.
- 7Systems Biomedical Informatics National Core Research Center, Division of Biomedical Informatics, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 1435179
- DOI: http://doi.org/10.5124/jkma.2012.55.8.729
Abstract
- Around the world electronic health records data are being shared and exchanged between two different systems for direct patient care, as well as for research, reimbursement, quality assurance, epidemiology, public health, and policy development. It is important to communicate the semantic meaning of the clinical data when exchanging electronic health records data. In order to achieve semantic interoperability of clinical data, it is important not only to specify clinical entries and documents and the structure of data in electronic health records, but also to use clinical terminology to describe clinical data. There are three types of clinical terminology: interface terminology to support a user-friendly structured data entry; reference terminology to store, retrieve, and analyze clinical data; and classification to aggregate clinical data for secondary use. In order to use electronic health records data in an efficient way, healthcare providers first need to record clinical content using a systematic and controlled interface terminology, then clinical content needs to be stored with reference terminology in a clinical data repository or data warehouse, and finally, the clinical content can be converted into a classification for reimbursement and statistical reporting. For electronic health records data collected at the point of care to be used for secondary purposes, it is necessary to map reference terminology with interface terminology and classification. It is necessary to adopt clinical terminology in electronic health records systems to ensure a high level of semantic interoperability.
Keyword
MeSH Terms
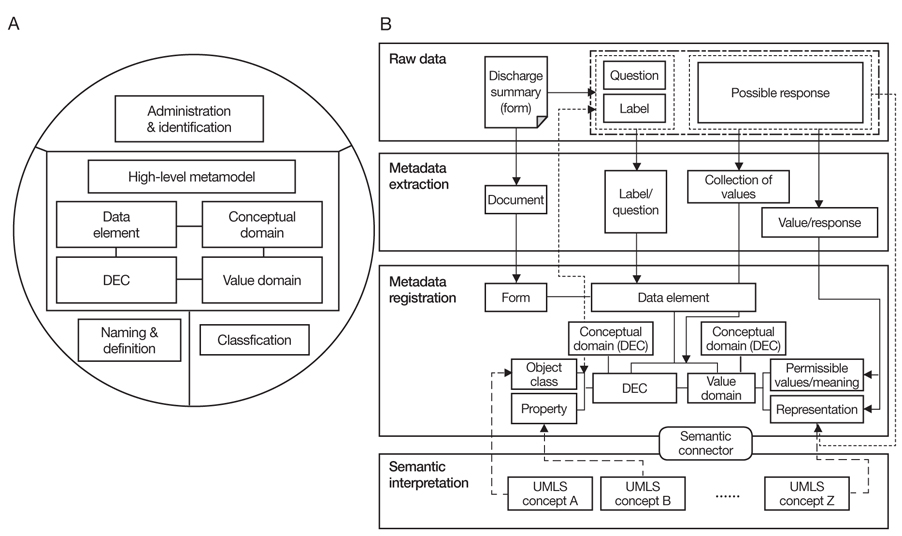
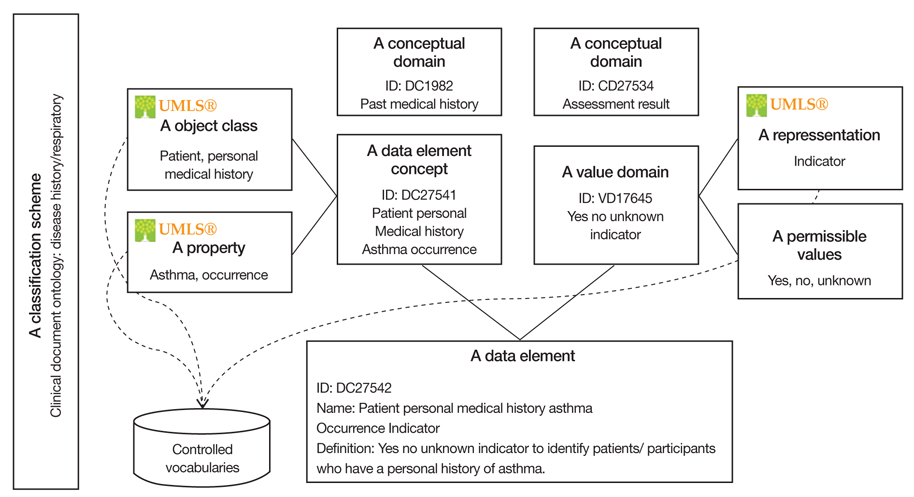
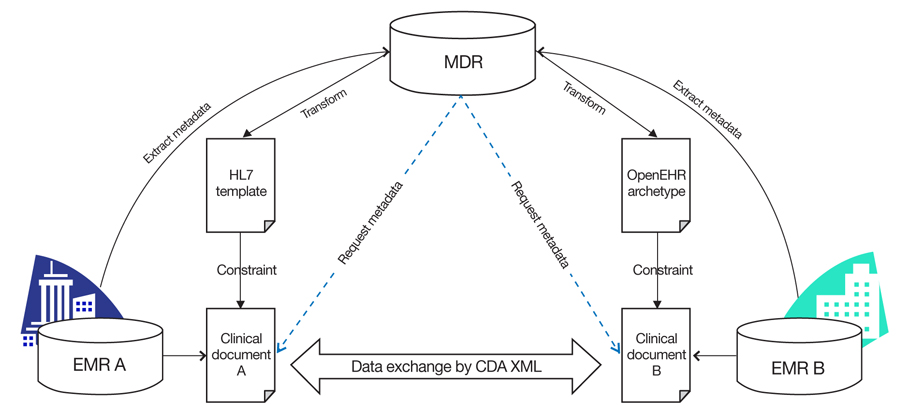
Figure
Cited by 2 articles
-
Health Avatar: An Informatics Platform for Personal and Private Big Data
Ju Han Kim
Healthc Inform Res. 2014;20(1):1-2. doi: 10.4258/hir.2014.20.1.1.CCR+: Metadata Based Extended Personal Health Record Data Model Interoperable with the ASTM CCR Standard
Yu Rang Park, Young Jo Yoon, Tae Hun Jang, Hwa Jeong Seo, Ju Han Kim
Healthc Inform Res. 2014;20(1):39-44. doi: 10.4258/hir.2014.20.1.39.
Reference
-
1. Dolin RH, Alschuler L, Boyer S, Beebe C, Behlen FM, Biron PV, Shabo Shvo A. HL7 clinical document architecture, release 2. J Am Med Inform Assoc. 2006. 13:30–39.2. American Society for Testing and Materials. Standard specification for continuity of care record (CCR) [Internet]. 2005. cited 2012 Jun 28. West Conshohocken: ASTM International;Available from: http://www.astm.org/Standards/E2369.htm.3. Clinical Data Interchange Standards Consortium. Operational data model [Internet]. 2012. cited 2012 Jun 28. Round Rock: Clinical Data Interchange Standards Consortium;Available from: http://www.cdisc.org/odm.4. Hurrell MJ, Monk TG, Nicol A, Norton AN, Reich DL, Walsh JL. Implementation of a standards-based anaesthesia record compliant with the health level 7 (HL7) clinical document architecture (CDA). J Clin Monit Comput. 2012. 26:295–304.5. Otter-Nickerson B. HIE: the interoperable way to deliver quality healthcare: finally, the concept of health information exchange is being more concretely defined and accepted by the healthcare community. Health Manag Technol. 2011. 32:19.6. Richesson RL, Krischer J. Data standards in clinical research: gaps, overlaps, challenges and future directions. J Am Med Inform Assoc. 2007. 14:687–696.7. Smith B, Ceusters W. HL7 RIM: an incoherent standard. Stud Health Technol Inform. 2006. 124:133–138.8. Humphreys BL, Lindberg DA, Schoolman HM, Barnett GO. The unified medical language system: an informatics research collaboration. J Am Med Inform Assoc. 1998. 5:1–11.9. Cote RA, Rothwell DJ, Palotay JL, Beckett RS, Brochu L. College of American Pathologists. American Veterinary Medical Association. The systematized nomenclature of human and veterinary medicine: SNOMED international. 1993. 3rd ed. Northfield: College of American Pathologists.10. Solbrig HR. Metadata and the reintegration of clinical information: ISO 11179. MD Comput. 2000. 17:25–28.
Article11. Agency for Healthcare Research and Quality. United States Health Information Knowledgebase [Internet]. 2012. cited 2012 Jun 18. Rockville: Agency for Healthcare Research and Quality;Available from: http://ushik.ahrq.gov.
Article12. Warzel DB, Andonaydis C, McCurry B, Chilukuri R, Ishmukhamedov S, Covitz P. Common data element (CDE) management and deployment in clinical trials. AMIA Annu Symp Proc. 2003. 1048.
Article13. International Organization for Standardization. International Electrotechnical Commission. Information technology: specification and standardization of data elements. Part 3. Basic attributes of data elements. 1994. Geneva: ISO/IEC 11179-3.
Article14. Fridsma DB, Evans J, Hastak S, Mead CN. The BRIDG project: a technical report. J Am Med Inform Assoc. 2008. 15:130–137.
Article15. Buetow KH, Niederhuber J. Infrastructure for a learning health care system: CaBIG. Health Aff (Millwood). 2009. 28:923–924.
Article16. Costa CM, Menarguez-Tortosa M, Fernandez-Breis JT. Clinical data interoperability based on archetype transformation. J Biomed Inform. 2011. 44:869–880.17. Clinical Data Interchange Standards Consortium. Clinical data acquisition standards harmonization [Internet]. 2012. cited 2012 Jun 28. Round Rock: Clinical Data Interchange Standards Consortium;Available from: http://www.cdisc.org/cdash.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- CCR+: Metadata Based Extended Personal Health Record Data Model Interoperable with the ASTM CCR Standard
- Development of Health Information Search Engine Based on Metadata and Ontology
- The Development of Clinical Document Standards for Semantic Interoperability in China
- Clinical Terminologies: A Solution for Semantic Interoperability
- Standardization for Promoting Interoperability of Healthcare Data: The Progression of the Korean Society of Radiology