J Bacteriol Virol.
2012 Mar;42(1):29-39. 10.4167/jbv.2012.42.1.29.
SmcR, the Quorum-sensing Master Regulator, Is Partially Involved in Temperature/Salinity-mediated Changes in Metalloprotease vvpE Expression in Vibrio vulnificus
- Affiliations
-
- 1Research Center for Resistant Cells, Chosun University Medical School Gwangju, Korea. shsin@chosun.ac.kr
- 2Department of Microbiology, Chosun University Medical School, Gwangju, Korea.
- KMID: 1434770
- DOI: http://doi.org/10.4167/jbv.2012.42.1.29
Abstract
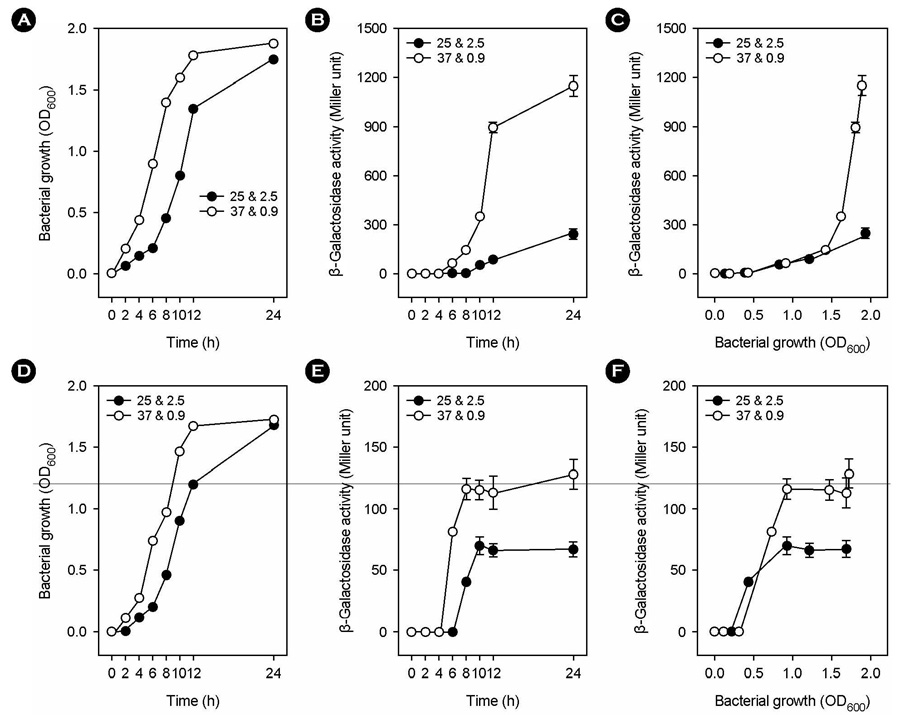
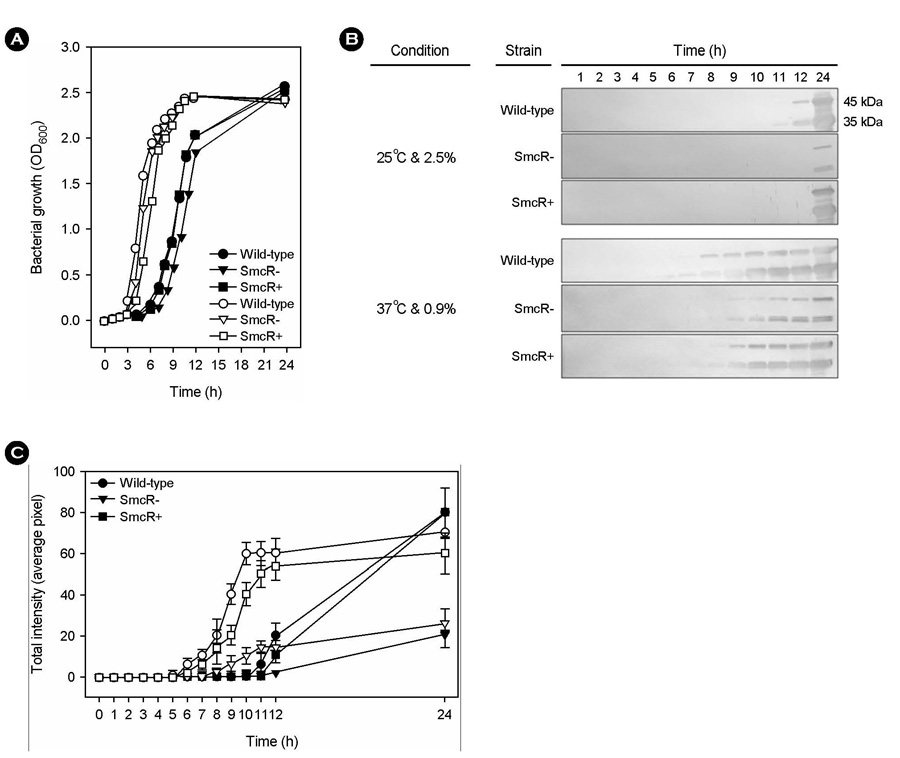
- Vibrio vulnificus, a gram-negative halophilic marine bacterium and opportunistic human pathogen, must withstand various environmental changes, especially simultaneous changes in temperature and salinity, from 25degrees C/2.5% to 37degrees C/0.9% (SCTS) upon entering the human body. In our previous study, SCTS stimulated vvpE expression even in the background of a mutation in luxS encoding LuxS enzyme for the biosynthesis of quorum-sensing (QS) signal molecule autoinducer-2 (AI-2), suggesting that the A1-2-mediated QS system is partially involved in the SCTS-mediated change of vvpE expression. In this study, we examined the effects of the QS master regulator SmcR on SCTS-mediated changes in vvpE expression and extracellular VvpE production. SCTS stimulated V. vulnificus growth, but with no increase in maximal growth levels. The SCTS-mediated prolongation of the stationary growth phase resulted in a significant increase in growth phase-dependent smcR and vvpE expressions. A mutation in smcR seriously repressed vvpE expression, but had no significant effect on V. vulnificus growth. However, the smcR mutation only partially attenuates SCTS-mediated changes in vvpE expression. These results indicate that SCTS stimulates the expressions of smcR and vvpE by stimulating V. vulnificus growth, and that SmcR is only partially involved in SCTS-mediated changes in vvpE expression.
Figure
Reference
-
1. Jones MK, Oliver JD. Vibrio vulnificus: disease and pathogenesis. Infect Immun. 2009. 77:1723–1733.2. Shao CP, Hor LI. Metalloprotease is not essential for Vibrio vulnificus virulence in mice. Infect Immun. 2000. 68:3569–3573.
Article3. Jeong HS, Lee MH, Lee KH, Park SJ, Choi SH. SmcR and cyclic AMP receptor protein coactivate Vibrio vulnificus vvpE encoding elastase through the RpoS-dependent promoter in a synergistic manner. J Biol Chem. 2003. 278:45072–45081.
Article4. Kim CM, Shin SH. Regulation of the Vibrio vulnificus vvpE expression by cyclic AMP-receptor protein and quorum-sensing regulator SmcR. Microb Pathog. 2010. 49:348–353.
Article5. Park J, Ryu SY, Kim CM, Shin SH. Two forms of Vibrio vulnificus metalloprotease VvpE are secreted via the type II general secretion system. J Microbiol. 2008. 46:338–343.
Article6. Hülsmann A, Rosche TM, Kong IS, Hassan HM, Beam DM, Oliver JD. RpoS-dependent stress response and exoenzyme production in Vibrio vulnificus. Appl Environ Microbiol. 2003. 69:6114–6120.
Article7. Kim CM, Chung YY, Shin SH. Iron differentially regulates gene expression and extracellular secretion of Vibrio vulnificus cytolysin-hemolysin. J Infect Dis. 2009. 200:582–589.
Article8. Redfield RJ. Is quorum sensing a side effect of diffusion sensing? Trends Microbiol. 2002. 10:365–370.
Article9. Roh JB, Lee MA, Lee HJ, Kim SM, Cho Y, Kim YJ, et al. Transcriptional regulatory cascade for elastase production in Vibrio vulnificus: LuxO activates luxT expression and LuxT represses smcR expression. J Biol Chem. 2006. 281:34775–34784.
Article10. Valiente E, Bruhn JB, Nielsen KF, Larsen JL, Roig FJ, Gram L, et al. Vibrio vulnificus produces quorum sensing signals of the AHL-class. FEMS Microbiol Ecol. 2009. 69:16–26.11. Defoirdt T, Boon N, Sorgeloos P, Verstraete W, Bossier P. Quorum sensing and quorum quenching in Vibrio harveyi: lessons learned from in vivo work. ISME J. 2008. 2:19–26.
Article12. Carnes EC, Lopez DM, Donegan NP, Cheung A, Gresham H, Timmins GS, et al. Confinement-induced quorum sensing of individual Staphylococcus aureus bacteria. Nat Chem Biol. 2010. 6:41–45.
Article13. McDougald D, Rice SA, Kjelleberg S. SmcR-dependent regulation of adaptive phenotypes in Vibrio vulnificus. J Bacteriol. 2001. 183:758–762.
Article14. Jones MK, Warner E, Oliver JD. Survival of and in situ gene expression by Vibrio vulnificus at varying salinities in estuarine environments. Appl Environ Microbiol. 2008. 74:182–187.
Article15. Shao CP, Hor LI. Regulation of metalloprotease gene expression in Vibrio vulnificus by a Vibrio harveyi LuxR homologue. J Bacteriol. 2001. 183:1369–1375.
Article16. Kawase T, Miyoshi S, Sultan Z, Shinoda S. Regulation system for protease production in Vibrio vulnificus. FEMS Microbiol Lett. 2004. 240:55–59.17. Kim CM, Shin SH. Change of Vibrio vulnificus Metalloprotease VvpE production by temperature and salinity. J Bacteriol Virol. 2011. 41:147–156.
Article18. Kim SY, Lee SE, Kim YR, Kim CM, Ryu PY, Choy HE, et al. Regulation of Vibrio vulnificus virulence by the LuxS quorum-sensing system. Mol Microbiol. 2003. 48:1647–1664.
Article19. Reddy GP, Hayat U, Abeygunawardana C, Fox C, Wright AC, Maneval DR jr, et al. Purification and determination of the structure of capsular polysaccharide of Vibrio vulnificus M06-24. J Bacteriol. 1992. 174:2620–2630.
Article20. Miller VL, Mekalanos JJ. A novel suicide vector and its use in construction of insertion mutations: Osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J Bacteriol. 1988. 170:2575–2583.
Article21. McGee K, Horstedt P, Milton DL. Identification and characterization of additional flagellin genes from Vibrio anguillarum. J Bacteriol. 1996. 178:5188–5198.
Article22. Farinha MA, Kropinski AM. Construction of broad-host-range plasmid vectors for easy visible selection and analysis of promoters. J Bacteriol. 1990. 172:3496–3499.
Article23. Staskawicz B, Dahlbeck D, Keen N, Napoli C. Molecular characterization of cloned avirulence genes from race 0 and race 1 of Pseudomonas syringae pv. Glycinea. J Bacteriol. 1987. 169:5789–5794.
Article24. Ditta G, Stanfield S, Corbin D, Helinski DR. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci U S A. 1980. 77:7347–7351.
Article25. Herrero M, de Lorenzo V, Timmis KN. Transposon vectors containing non-antibiotic resistance selection markers for cloning and stable chromosomal insertion of foreign genes in gram-negative bacteria. J Bacteriol. 1990. 172:6557–6567.
Article26. Miller JH. A short course in bacterial genetics. 1992. New York: Cold Spring Harbor, Cold Spring Harbor Laboratory Press.27. Kim CM, Park RY, Chun HJ, Kim SY, Rhee JH, Shin SH. Vibrio vulnificus metalloprotease VvpE is essentially required for swarming. FEMS Microbiol Lett. 2007. 269:170–179.
Article28. Fraser GM, Hughes C. Swarming motility. Curr Opin Microbiol. 1999. 2:630–635.
Article29. Walker KE, Moghaddame-Jafari S, Lockatell CV, Johnson D, Belas R. ZapA, the IgA-degrading metalloprotease of Proteus mirabilis, is a virulence factor expressed specifically in swarmer cells. Mol Microbiol. 1999. 32:825–836.
Article30. Lee JH, Rhee JE, Park U, Ju HM, Lee BC, Kim TS, et al. Identification and functional analysis of Vibrio vulnificus SmcR, a novel global regulator. J Microbiol Biotechnol. 2007. 17:325–334.31. Vendeville A, Winzer K, Heurlier K, Tang CM, Hardie KR. Making 'sense' of metabolism: autoinducer-2, LuxS and pathogenic bacteria. Nat Rev Microbiol. 2005. 3:383–396.
Article32. Konkel ME, Tilly K. Temperature-regulated expression of bacterial virulence genes. Microbes Infect. 2000. 2:157–166.
Article33. Maurelli AT, Blackmon B, Curtiss R 3rd. Temperature-dependent expression of virulence genes in Shigella species. Infect Immun. 1984. 43:195–201.
Article34. Lee HJ, Park SJ, Choi SH, Lee KH. Vibrio vulnificus rpoS expression is repressed by direct binding of cAMP-cAMP receptor protein complex to its two promoter regions. J Biol Chem. 2008. 283:30438–30450.
Article35. Kim CM, Park RY, Park JH, Sun HY, Bai YH, Ryu PY, et al. Vibrio vulnificus vulnibactin, but not metalloprotease VvpE, is essentially required for iron-uptake from human holotransferrin. Biol Pharm Bull. 2006. 29:911–918.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Change of Vibrio vulnificus Metalloprotease VvpE Production by Temperature and Salinity
- Quorum Sensing System and Virulence Regulation in Vibrio vulnificus
- Effect of Salinity, Temperature, and Glucose on the Production of Vibrio vulnificus Hemolysin
- Isolation of Vibrio vulnificus from Seawater and Emerging Vibrio vulnificus Septicemia on Jeju Island
- Effects of Environmental Sea Water Factors on the Isolation of Vibrio vulnificus in the Western Coastal Area of Korea