Korean J Radiol.
2013 Feb;14(1):61-69. 10.3348/kjr.2013.14.1.61.
Diffusion-Weighted Magnetic Resonance Imaging for the Evaluation of Prostate Cancer: Optimal B Value at 3T
- Affiliations
-
- 1Department of Radiology and Center for Imaging Science, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul 135-710, Korea. chankyokim@skku.edu
- 2Department of Pathology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul 135-710, Korea.
- 3Department of Radiology, Mayo Clinic College of Medicine, Rochester MN55905, USA.
- KMID: 1430045
- DOI: http://doi.org/10.3348/kjr.2013.14.1.61
Abstract
OBJECTIVE
To retrospectively determine the optimal b value of diffusion-weighted imaging (DWI) for predicting the presence of localized prostate cancer, and to evaluate the utility of DWI under different b values in differentiating between cancers and benign prostatic tissues.
MATERIALS AND METHODS
Eighty patients with suspected prostate cancer underwent MRI including DWI at 3T, followed by radical prostatectomy. DWI was examined under different b values. Apparent diffusion coefficient (ADC) maps were generated by using b = 0, and other b values of 300, 700, 1000 or 2000 s/mm2. For predicting the presence of cancers, four different ADC maps were analyzed independently by two blinded readers. ADCs were measured in benign and malignant tissues.
RESULTS
For predicting the presence of 110 prostate cancers, the sensitivity and area under the curve (AUC) for an experienced reader was significantly greater at b = 1000 (85% and 0.91) than b = 300, 700 or 2000 s/mm2 (p < 0.01). For a less-experienced reader, the AUC was significantly greater at b = 700, 1000 or 2000 than b = 300 s/mm2 (p < 0.01). Mean ADCs of the cancers in sequence from b = 300 to 2000 s/mm2 were 1.33, 1.03, 0.88 and 0.68 x 10(-3) mm2/s, which were significantly lower than those of benign tissues (p < 0.001).
CONCLUSION
The optimal b value for 3T DWI for predicting the presence of prostate cancer may be 1000 s/mm2.
MeSH Terms
-
Aged
Aged, 80 and over
Area Under Curve
Biopsy
Diagnosis, Differential
Diffusion Magnetic Resonance Imaging/*methods
Humans
Male
Middle Aged
Neoplasm Staging
Predictive Value of Tests
Prostate-Specific Antigen/blood
Prostatectomy
Prostatic Neoplasms/*diagnosis/pathology/surgery
Retrospective Studies
Sensitivity and Specificity
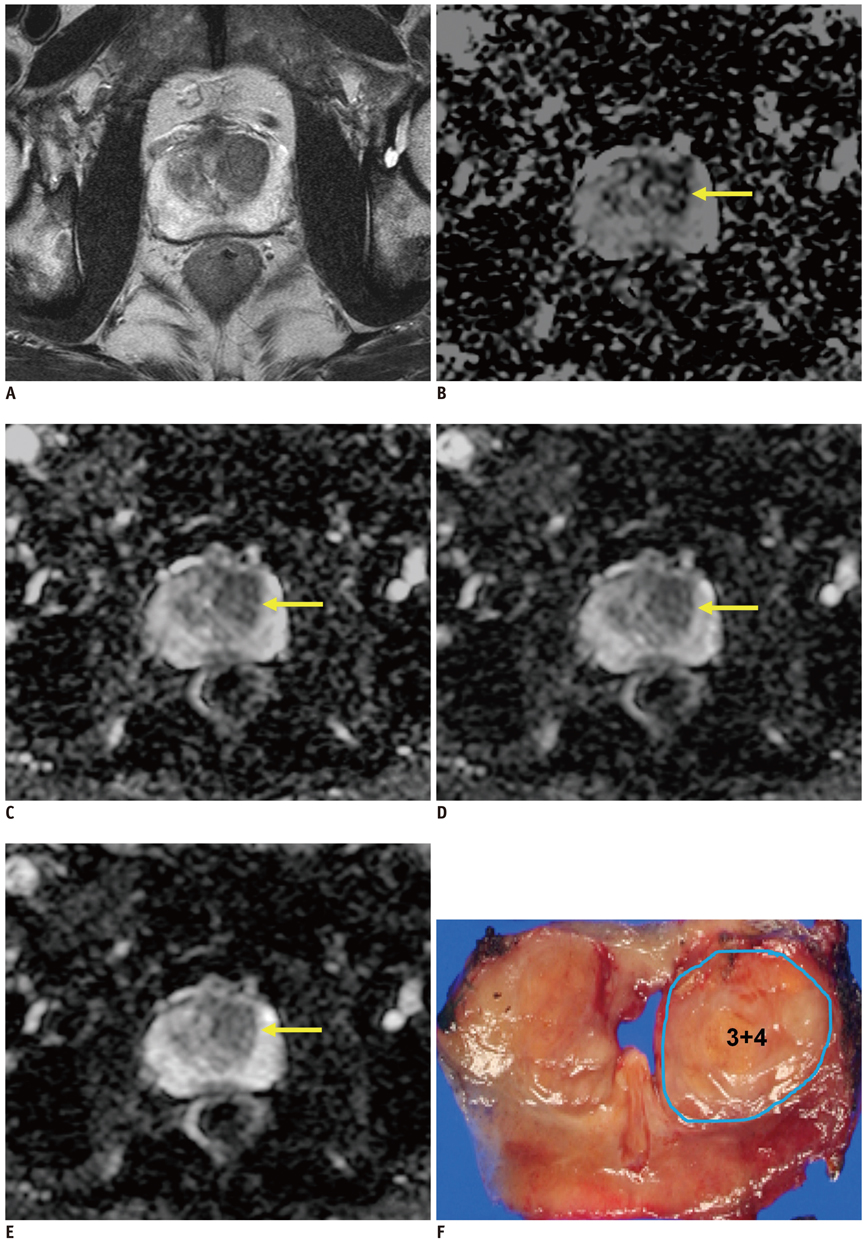
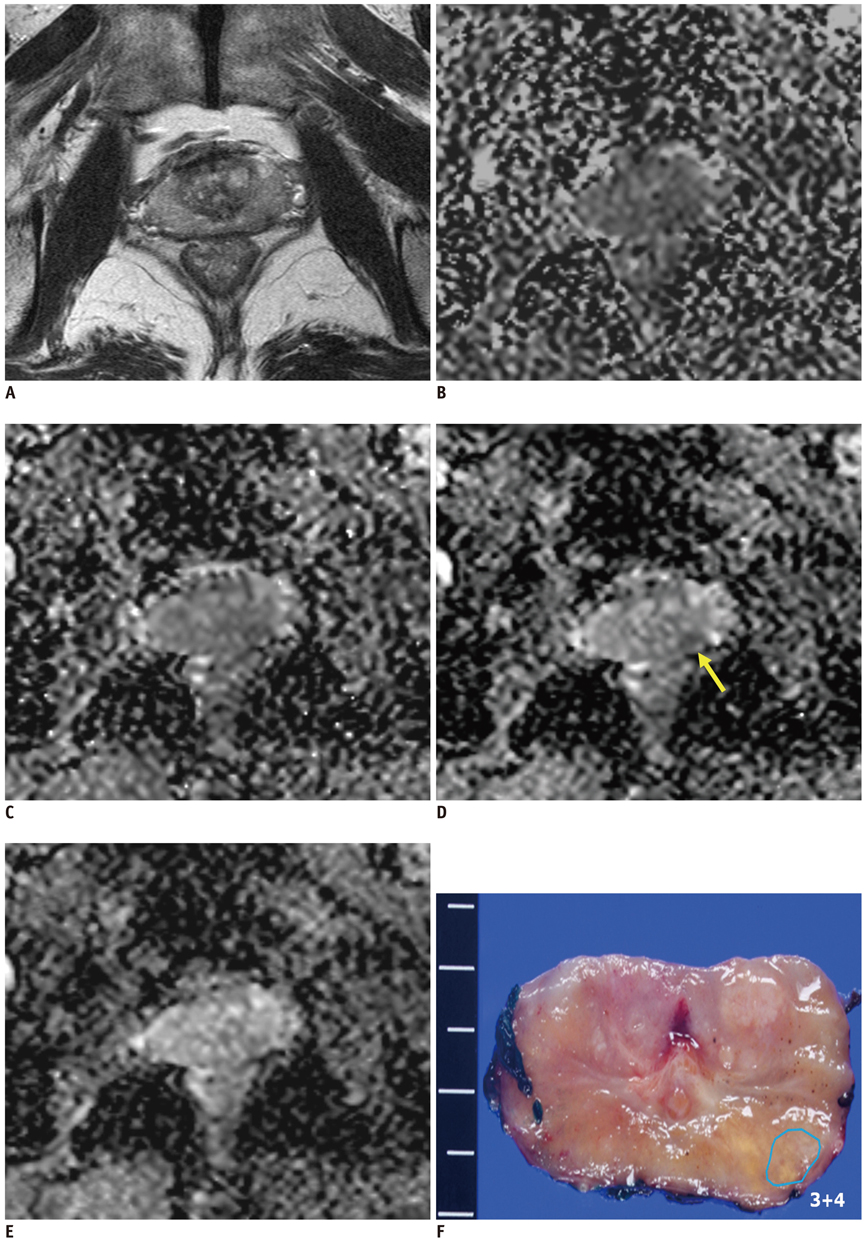
Figure
Cited by 1 articles
-
Computed Diffusion-Weighted Imaging in Prostate Cancer: Basics, Advantages, Cautions, and Future Prospects
Yoshiko R. Ueno, Tsutomu Tamada, Satoru Takahashi, Utaru Tanaka, Keitaro Sofue, Tomonori Kanda, Munenobu Nogami, Yoshiharu Ohno, Nobuyuki Hinata, Masato Fujisawa, Takamichi Murakami
Korean J Radiol. 2018;19(5):832-837. doi: 10.3348/kjr.2018.19.5.832.
Reference
-
1. Kim CK, Park BK, Kim B. Diffusion-weighted MRI at 3 T for the evaluation of prostate cancer. AJR Am J Roentgenol. 2010. 194:1461–1469.2. Yakar D, Hambrock T, Huisman H, Hulsbergen-van de Kaa CA, van Lin E, Vergunst H, et al. Feasibility of 3T dynamic contrast-enhanced magnetic resonance-guided biopsy in localizing local recurrence of prostate cancer after external beam radiation therapy. Invest Radiol. 2010. 45:121–125.3. Turkbey B, Shah VP, Pang Y, Bernardo M, Xu S, Kruecker J, et al. Is apparent diffusion coefficient associated with clinical risk scores for prostate cancers that are visible on 3-T MR images? Radiology. 2011. 258:488–495.4. Kim CK, Park BK, Kim B. High-b-value diffusion-weighted imaging at 3 T to detect prostate cancer: comparisons between b values of 1,000 and 2,000 s/mm2. AJR Am J Roentgenol. 2010. 194:W33–W37.5. Katahira K, Takahara T, Kwee TC, Oda S, Suzuki Y, Morishita S, et al. Ultra-high-b-value diffusion-weighted MR imaging for the detection of prostate cancer: evaluation in 201 cases with histopathological correlation. Eur Radiol. 2011. 21:188–196.6. Pickles MD, Gibbs P, Sreenivas M, Turnbull LW. Diffusion-weighted imaging of normal and malignant prostate tissue at 3.0T. J Magn Reson Imaging. 2006. 23:130–134.7. Haider MA, van der Kwast TH, Tanguay J, Evans AJ, Hashmi AT, Lockwood G, et al. Combined T2-weighted and diffusion-weighted MRI for localization of prostate cancer. AJR Am J Roentgenol. 2007. 189:323–328.8. deSouza NM, Riches SF, Vanas NJ, Morgan VA, Ashley SA, Fisher C, et al. Diffusion-weighted magnetic resonance imaging: a potential non-invasive marker of tumour aggressiveness in localized prostate cancer. Clin Radiol. 2008. 63:774–782.9. Kim CK, Park BK, Lee HM, Kwon GY. Value of diffusion-weighted imaging for the prediction of prostate cancer location at 3T using a phased-array coil: preliminary results. Invest Radiol. 2007. 42:842–847.10. Jager GJ, Ruijter ET, van de Kaa CA, de la Rosette JJ, Oosterhof GO, Thornbury JR, et al. Dynamic TurboFLASH subtraction technique for contrast-enhanced MR imaging of the prostate: correlation with histopathologic results. Radiology. 1997. 203:645–652.11. Oto A, Kayhan A, Jiang Y, Tretiakova M, Yang C, Antic T, et al. Prostate cancer: differentiation of central gland cancer from benign prostatic hyperplasia by using diffusion-weighted and dynamic contrast-enhanced MR imaging. Radiology. 2010. 257:715–723.12. Heo SH, Jeong YY, Shin SS, Kim JW, Lim HS, Lee JH, et al. Apparent diffusion coefficient value of diffusion-weighted imaging for hepatocellular carcinoma: correlation with the histologic differentiation and the expression of vascular endothelial growth factor. Korean J Radiol. 2010. 11:295–303.13. Moon WJ, Lee MH, Chung EC. Diffusion-weighted imaging with sensitivity encoding (SENSE) for detecting cranial bone marrow metastases: comparison with T1-weighted images. Korean J Radiol. 2007. 8:185–191.14. Saritas EU, Lee JH, Nishimura DG. SNR dependence of optimal parameters for apparent diffusion coefficient measurements. IEEE Trans Med Imaging. 2011. 30:424–437.15. Kitajima K, Kaji Y, Kuroda K, Sugimura K. High b-value diffusion-weighted imaging in normal and malignant peripheral zone tissue of the prostate: effect of signal-to-noise ratio. Magn Reson Med Sci. 2008. 7:93–99.16. Rosenkrantz AB, Kong X, Niver BE, Berkman DS, Melamed J, Babb JS, et al. Prostate cancer: comparison of tumor visibility on trace diffusion-weighted images and the apparent diffusion coefficient map. AJR Am J Roentgenol. 2011. 196:123–129.17. DeLano MC, Cooper TG, Siebert JE, Potchen MJ, Kuppusamy K. High-b-value diffusion-weighted MR imaging of adult brain: image contrast and apparent diffusion coefficient map features. AJNR Am J Neuroradiol. 2000. 21:1830–1836.18. Shinmoto H, Oshio K, Tanimoto A, Higuchi N, Okuda S, Kuribayashi S, et al. Biexponential apparent diffusion coefficients in prostate cancer. Magn Reson Imaging. 2009. 27:355–359.19. Hambrock T, Somford DM, Huisman HJ, van Oort IM, Witjes JA, Hulsbergen-van de Kaa CA, et al. Relationship between Apparent Diffusion Coefficients at 3.0-T MR Imaging and Gleason Grade in Peripheral Zone Prostate Cancer. Radiology. 2011. [Epub ahead of print].
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- RE: Diffusion-Weighted Imaging of Prostate Cancer: How Can We Use It Accurately?
- Prostate Imaging Reporting and Data System (PI-RADS) v 2.1: Overview and Critical Points
- Multidisciplinary Functional MR Imaging for Prostate Cancer
- Medical imaging of prostate cancer
- Computed Diffusion-Weighted Imaging in Prostate Cancer: Basics, Advantages, Cautions, and Future Prospects