Korean J Radiol.
2013 Feb;14(1):45-50. 10.3348/kjr.2013.14.1.45.
Establishment of a Protocol for Determining Gastrointestinal Transit Time in Mice Using Barium and Radiopaque Markers
- Affiliations
-
- 1Department of Radiology, Chonnam National University Hwasun Hospital, Chonnam National University Medical School, Hwasun 519-763, Korea. yjeong@jnu.ac.kr
- 2Department of Physiology, Chonnam National University Medical School, Gwangju 501-757, Korea.
- 3Department of Physiology, College of Medicine, Chosun University, Gwangju 501-717, Korea.
- KMID: 1430043
- DOI: http://doi.org/10.3348/kjr.2013.14.1.45
Abstract
OBJECTIVE
The purpose of this study was to establish a minimally invasive and reproducible protocol for estimating the gastrointestinal (GI) transit time in mice using barium and radiopaque markers.
MATERIALS AND METHODS
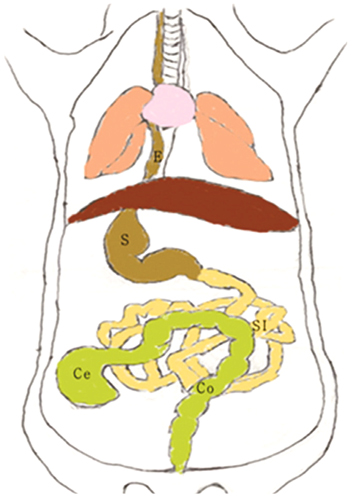
Twenty 5- to 6-week-old Balb/C female mice weighing 19-21 g were used. The animals were divided into three groups: two groups that received loperamide and a control group. The control group (n = 10) animals were administered physiological saline (1.5 mL/kg) orally. The loperamide group I (n = 10) and group II (n = 10) animals were administered 5 mg/kg and 10 mg/kg loperamide orally, respectively. Thirty minutes after receiving the saline or loperamide, the mice was administered 80 microL of barium solution and six iron balls (0.5 mm) via the mouth and the upper esophagus by gavage, respectively. Afterwards, the mice were continuously monitored with fluoroscopic imaging in order to evaluate the swallowing of the barium solution and markers. Serial fluoroscopic images were obtained at 5- or 10-min intervals until all markers had been excreted from the anal canal. For analysis, the GI transit times were subdivided into intestinal transit times (ITTs) and colon transit times (CTTs).
RESULTS
The mean ITT was significantly longer in the loperamide groups than in the control group (p < 0.05). The mean ITT in loperamide group II (174.5 +/- 32.3) was significantly longer than in loperamide group I (133.2 +/- 24.2 minute) (p < 0.05). The mean CTT was significantly longer in loperamide group II than in the control group (p < 0.05). Also, no animal succumbed to death after the experimental procedure.
CONCLUSION
The protocol for our study using radiopaque markers and barium is reproducible and minimally invasive in determining the GI transit time of the mouse model.
MeSH Terms
-
Analysis of Variance
Animals
Barium Sulfate/pharmacology
Contrast Media/administration & dosage
Female
Fluoroscopy
Gastrointestinal Transit/*physiology
Iron
Loperamide/administration & dosage
Mice
Mice, Inbred BALB C
Microscopy, Electron, Scanning
Prostheses and Implants
Reproducibility of Results
Sodium Chloride/administration & dosage
Surface Properties
Figure
Reference
-
1. Parkman HP, Orr WC. The gastrointestinal motility laboratory. Gastrointest Endosc Clin N Am. 2009. 19:171–184. viii2. Merwid-Lad A, Trocha M, Ksiadzyna D, Sozanski T, Szelag A. Animals models for the gastrointestinal motility evaluation. Gastroenterol Pol. 2009. 16:201–206.3. Lester NV, Roberts GD, Newell SM, Graham JP, Hartless CS. Assessment of barium impregnated polyethylene spheres (BIPS) as a measure of solid-phase gastric emptying in normal dogs--comparison to scintigraphy. Vet Radiol Ultrasound. 1999. 40:465–471.4. Weber M, Stambouli F, Martin L, Dumon H, Biourge V, Nguyen P. Gastrointestinal transit of solid radiopaque markers in large and giant breed growing dogs. J Anim Physiol Anim Nutr (Berl). 2001. 85:242–250.5. Chandler ML, Guilford G, Lawoko CR. Radiopaque markers to evaluate gastric emptying and small intestinal transit time in healthy cats. J Vet Intern Med. 1997. 11:361–364.6. Haruta S, Kawai K, Jinnouchi S, Ogawara KI, Higaki K, Tamura S, et al. Evaluation of absorption kinetics of orally administered theophylline in rats based on gastrointestinal transit monitoring by gamma scintigraphy. J Pharm Sci. 2001. 90:464–473.7. Marona HR, Lucchesi MB. Protocol to refine intestinal motility test in mice. Lab Anim. 2004. 38:257–260.8. Sparkes AH, Papasouliotis K, Barr FJ, Gruffydd-Jones TJ. Reference ranges for gastrointestinal transit of barium-impregnated polyethylene spheres in healthy cats. J Small Anim Pract. 1997. 38:340–343.9. Campbell JL, Williams CV, Eisemann JH. Characterizing gastrointestinal transit time in four lemur species using barium-impregnated polyethylene spheres (BIPS). Am J Primatol. 2004. 64:309–321.10. Hinton JM, Lennard-Jones JE, Young AC. A ne method for studying gut transit times using radioopaque markers. Gut. 1969. 10:842–847.11. Mittelstadt SW, Hemenway CL, Spruell RD. Effects of fasting on evaluation of gastrointestinal transit with charcoal meal. J Pharmacol Toxicol Methods. 2005. 52:154–158.12. Tan-No K, Niijima F, Nakagawasai O, Sato T, Satoh S, Tadano T. Development of tolerance to the inhibitory effect of loperamide on gastrointestinal transit in mice. Eur J Pharm Sci. 2003. 20:357–363.13. Ogata N, Ataka K, Morino H, Shibata T. Effect of wood creosote and loperamide on propulsive motility of mouse colon and small intestine. Pharmacology. 1999. 59:212–220.14. Torjman MC, Joseph JI, Munsick C, Morishita M, Grunwald Z. Effects of isoflurane on gastrointestinal motility after brief exposure in rats. Int J Pharm. 2005. 294:65–71.15. Lee H, Dellatore SM, Miller WM, Messersmith PB. Mussel-inspired surface chemistry for multifunctional coatings. Science. 2007. 318:426–430.16. BioForum. Available at: http://www.protocol-online.org/forums/topic/7182-mouse-liver-anatomy/.17. Saphier S, Rosner A, Brandeis R, Karton Y. Gastro intestinal tracking and gastric emptying of solid dosage forms in rats using X-ray imaging. Int J Pharm. 2010. 388:190–195.18. Rao SS, Camilleri M, Hasler WL, Maurer AH, Parkman HP, Saad R, et al. Evaluation of gastrointestinal transit in clinical practice: position paper of the American and European Neurogastroenterology and Motility Societies. Neurogastroenterol Motil. 2011. 23:8–23.19. Parrish CR. Opioid analgesics and the gastrointestinal tract. Pract Gastroenterol. 2008. 64:37–50.20. Szczesny G, Veihelmann A, Massberg S, Nolte D, Messmer K. Long-term anaesthesia using inhalatory isoflurane in different strains of mice-the haemodynamic effects. Lab Anim. 2004. 38:64–69.21. Hildebrandt IJ, Su H, Weber WA. Anesthesia and other considerations for in vivo imaging of small animals. ILAR J. 2008. 49:17–26.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Usefulness of Colonic Transit Time Measurement in Chronic Constipation
- Regional Gastrointestinal Transit Times in Patients With Carcinoid Diarrhea: Assessment With the Novel 3D-Transit System
- Segmental Colon Transit Time with Radiopaque Markers in a Delayed-release Capsule
- Rectosigmoid Localization of Radiopaque Markers for Identifying Defecation Disorders in Patients With Chronic Constipation: A Retrospective Cohort Study
- Two Cases of Slow Transit Constipation Associated with Delayed Gastric Emptying Treated by Total Abdominal Colectomy