Chonnam Med J.
2013 Aug;49(2):69-74. 10.4068/cmj.2013.49.2.69.
Indirect Radionuclide Coronary Angiography to Evaluate Gradients of Myocardial Blood Flow and Flow Reserve Through Coronary Stenosis Using N-13 Ammonia PET/CT
- Affiliations
-
- 1Department of Nuclear Medicine, Chonnam National University Medical School, Gwangju, Korea. hsbom@jnu.ac.kr
- 2Department of Cardiology, Chonnam National University Medical School, Gwangju, Korea.
- KMID: 1428265
- DOI: http://doi.org/10.4068/cmj.2013.49.2.69
Abstract
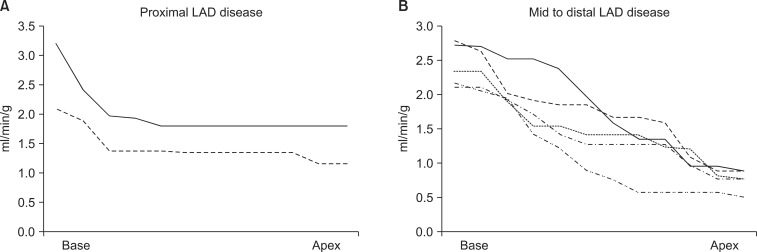
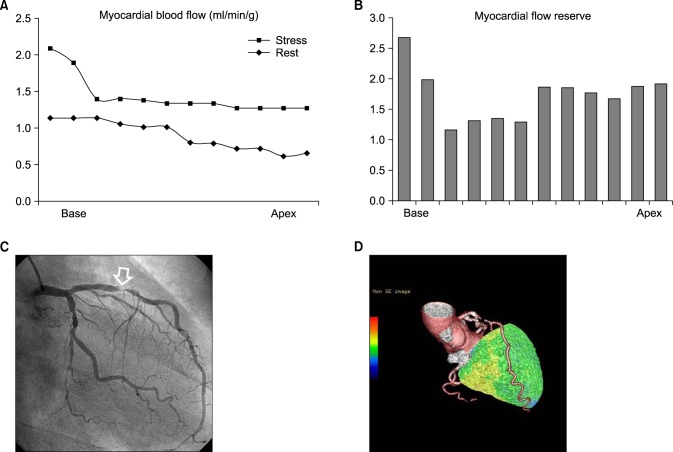
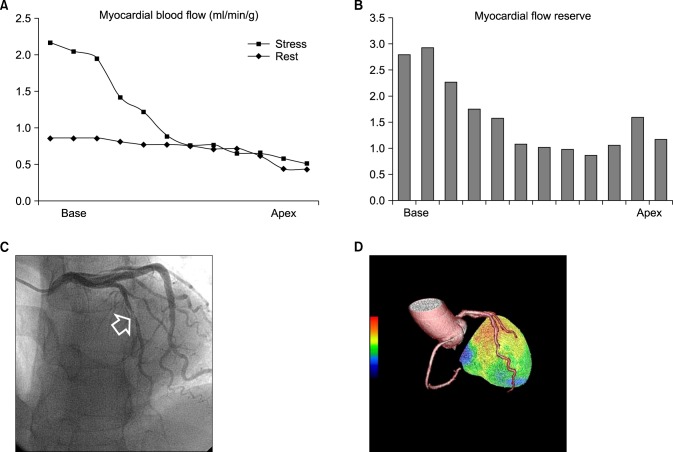
- Although quantitative evaluation of myocardial blood flow (MBF) and myocardial flow reserve (MFR) has been perceived as an attractive advantage of positron emission tomography (PET) over other cardiac imaging technologies, application of the information to specific coronary lesions is a difficult task for nuclear cardiologists. We hypothesized that changes in MBF and MFR over a coronary lesion could be identified by use of a hybrid technology of CT coronary angiography (CTCA) and N-13 ammonia PET. To evaluate this hypothesis, we measured the gradient of MBF and MFR through coronary stenosis in seven patients (M:F=3:4, median age 56 years) with coronary artery disease who underwent N-13 ammonia PET, CTCA, and interventional coronary angiography. Two patients had proximal left anterior descending (LAD) coronary artery disease and five patients had mid to distal LAD disease. Mean global stress and rest MBF were 2.62+/-0.58 and 1.03+/-0.19 ml/min/g, respectively. Mean global MFR was 2.6+/-0.73. Regional stress and rest MBF in the LAD territory were 2.36+/-0.75 and 0.96+/-0.21 ml/min/g, respectively. Regional MFR in the LAD territory was 2.55+/-0.83 ml/min/g. Stress MBF changed dramatically according to the location of coronary stenosis. It dropped acutely in proximal lesions, whereas it diminished gradually in mid to distal lesions. In conclusion, by use of a hybrid technology of CTCA and PET, it was feasible to make a direct correlation of coronary lesions with the gradient of MFR and CFR through coronary stenosis, which indicated the severity of the coronary lesion. We named this technique indirect radionuclide coronary angiography.
MeSH Terms
Figure
Reference
-
1. Ghosh N, Rimoldi OE, Beanlands RS, Camici PG. Assessment of myocardial ischaemia and viability: role of positron emission tomography. Eur Heart J. 2010; 31:2984–2995. PMID: 20965888.2. Kaufmann PA, Camici PG. Myocardial blood flow measurement by PET: technical aspects and clinical applications. J Nucl Med. 2005; 46:75–88. PMID: 15632037.3. Lima RS, Watson DD, Goode AR, Siadaty MS, Ragosta M, Beller GA, et al. Incremental value of combined perfusion and function over perfusion alone by gated SPECT myocardial perfusion imaging for detection of severe three-vessel coronary artery disease. J Am Coll Cardiol. 2003; 42:64–70. PMID: 12849661.4. Fiechter M, Ghadri JR, Gebhard C, Fuchs TA, Pazhenkottil AP, Nkoulou RN, et al. Diagnostic value of 13N-ammonia myocardial perfusion PET: added value of myocardial flow reserve. J Nucl Med. 2012; 53:1230–1234. PMID: 22776752.5. Herzog BA, Husmann L, Valenta I, Gaemperli O, Siegrist PT, Tay FM, et al. Long-term prognostic value of 13N-ammonia myocardial perfusion positron emission tomography added value of coronary flow reserve. J Am Coll Cardiol. 2009; 54:150–156. PMID: 19573732.6. Fukushima K, Javadi MS, Higuchi T, Lautamäki R, Merrill J, Nekolla SG, et al. Prediction of short-term cardiovascular events using quantification of global myocardial flow reserve in patients referred for clinical 82Rb PET perfusion imaging. J Nucl Med. 2011; 52:726–732. PMID: 21498538.7. Ziadi MC, Dekemp RA, Williams KA, Guo A, Chow BJ, Renaud JM, et al. Impaired myocardial flow reserve on rubidium-82 positron emission tomography imaging predicts adverse outcomes in patients assessed for myocardial ischemia. J Am Coll Cardiol. 2011; 58:740–748. PMID: 21816311.8. Henzlova MJ, Cerqueira MD, Mahmarian JJ, Yao SS. Quality Assurance Committee of the American Society of Nuclear Cardiology. Stress protocols and tracers. J Nucl Cardiol. 2006; 13:e80–e90. PMID: 17174798.9. Lee BI, Kim KH, Kim JY, Kim SJ, Lee JS, Min JJ, et al. Correlation between semiquantitative myocardial perfusion score and absolute myocardial blood flow in 13N-ammonia PET. Nucl Med Mol Imaging. 2007; 41:194–200.10. Muzik O, Beanlands RS, Hutchins GD, Mangner TJ, Nguyen N, Schwaiger M. Validation of nitrogen-13-ammonia tracer kinetic model for quantification of myocardial blood flow using PET. J Nucl Med. 1993; 34:83–91. PMID: 8418276.11. Hutchins GD, Schwaiger M, Rosenspire KC, Krivokapich J, Schelbert H, Kuhl DE. Noninvasive quantification of regional blood flow in the human heart using N-13 ammonia and dynamic positron emission tomographic imaging. J Am Coll Cardiol. 1990; 15:1032–1042. PMID: 2312957.12. Chow BJ, Abraham A, Wells GA, Chen L, Ruddy TD, Yam Y, et al. Diagnostic accuracy and impact of computed tomographic coronary angiography on utilization of invasive coronary angiography. Circ Cardiovasc Imaging. 2009; 2:16–23. PMID: 19808560.13. Chow BJ, Wells GA, Chen L, Yam Y, Galiwango P, Abraham A, et al. Prognostic value of 64-slice cardiac computed tomography severity of coronary artery disease, coronary atherosclerosis, and left ventricular ejection fraction. J Am Coll Cardiol. 2010; 55:1017–1028. PMID: 20202518.14. Hoffmann U, Moselewski F, Cury RC, Ferencik M, Jang IK, Diaz LJ, et al. Predictive value of 16-slice multidetector spiral computed tomography to detect significant obstructive coronary artery disease in patients at high risk for coronary artery disease: patient-versus segment-based analysis. Circulation. 2004; 110:2638–2643. PMID: 15492297.15. Gaemperli O, Schepis T, Kalff V, Namdar M, Valenta I, Stefani L, et al. Validation of a new cardiac image fusion software for three-dimensional integration of myocardial perfusion SPECT and stand-alone 64-slice CT angiography. Eur J Nucl Med Mol Imaging. 2007; 34:1097–1106. PMID: 17245532.16. Tonino PA, De Bruyne B, Pijls NH, Siebert U, Ikeno F, van' t Veer M, et al. FAME Study Investigators. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009; 360:213–224. PMID: 19144937.17. Pijls NH. Fractional flow reserve to guide coronary revascularization. Circ J. 2013; 77:561–569. PMID: 23420635.18. Nandalur KR, Dwamena BA, Choudhri AF, Nandalur SR, Reddy P, Carlos RC. Diagnostic performance of positron emission tomography in the detection of coronary artery disease: a meta-analysis. Acad Radiol. 2008; 15:444–451. PMID: 18342769.19. Schindler TH, Facta AD, Prior JO, Cadenas J, Zhang XL, Li Y, et al. Structural alterations of the coronary arterial wall are associated with myocardial flow heterogeneity in type 2 diabetes mellitus. Eur J Nucl Med Mol Imaging. 2009; 36:219–229. PMID: 18704406.20. Kaufmann PA, Gnecchi-Ruscone T, Yap JT, Rimoldi O, Camici PG. Assessment of the reproducibility of baseline and hyperemic myocardial blood flow measurements with 15O-labeled water and PET. J Nucl Med. 1999; 40:1848–1856. PMID: 10565780.21. Wyss CA, Koepfli P, Mikolajczyk K, Burger C, von Schulthess GK, Kaufmann PA. Bicycle exercise stress in PET for assessment of coronary flow reserve: repeatability and comparison with adenosine stress. J Nucl Med. 2003; 44:146–154. PMID: 12571202.22. Austin RE Jr, Aldea GS, Coggins DL, Flynn AE, Hoffman JI. Profound spatial heterogeneity of coronary reserve. Discordance between patterns of resting and maximal myocardial blood flow. Circ Res. 1990; 67:319–331. PMID: 2376074.23. Chareonthaitawee P, Kaufmann PA, Rimoldi O, Camici PG. Heterogeneity of resting and hyperemic myocardial blood flow in healthy humans. Cardiovasc Res. 2001; 50:151–161. PMID: 11282088.24. Javadi MS, Lautamäki R, Merrill J, Voicu C, Epley W, McBride G, et al. Definition of vascular territories on myocardial perfusion images by integration with true coronary anatomy: a hybrid PET/CT analysis. J Nucl Med. 2010; 51:198–203. PMID: 20080895.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Evaluation of Myocardial Blood Flow and Coronary Flow Reserve Using Positron Emission Tomography
- A Case of Successful Percutaneous Coronary Intervention by Fractional Flow Reserve and 13N-Ammonia Positron Emission Tomography
- Coronary Flow Velocity Pattern in Patients with Myocardial Bridging of Coronary Artery
- Myocardial Contrast Echocardiography for the Assessment of Coronary Blood Flow Reserve
- Evaluation of Myocardial Ischemia Using Coronary Computed Tomography Angiography in Patients with Stable Angina