Korean J Perinatol.
2013 Mar;24(1):1-10. 10.14734/kjp.2013.24.1.1.
Neonatal Pulmonary Hypertension
- Affiliations
-
- 1Department of Pediatrics, Ilsan Paik Hospital, College of Medicine, Inje University, Ilsan, Korea. jhhwang@paik.ac.kr
- KMID: 1427065
- DOI: http://doi.org/10.14734/kjp.2013.24.1.1
Abstract
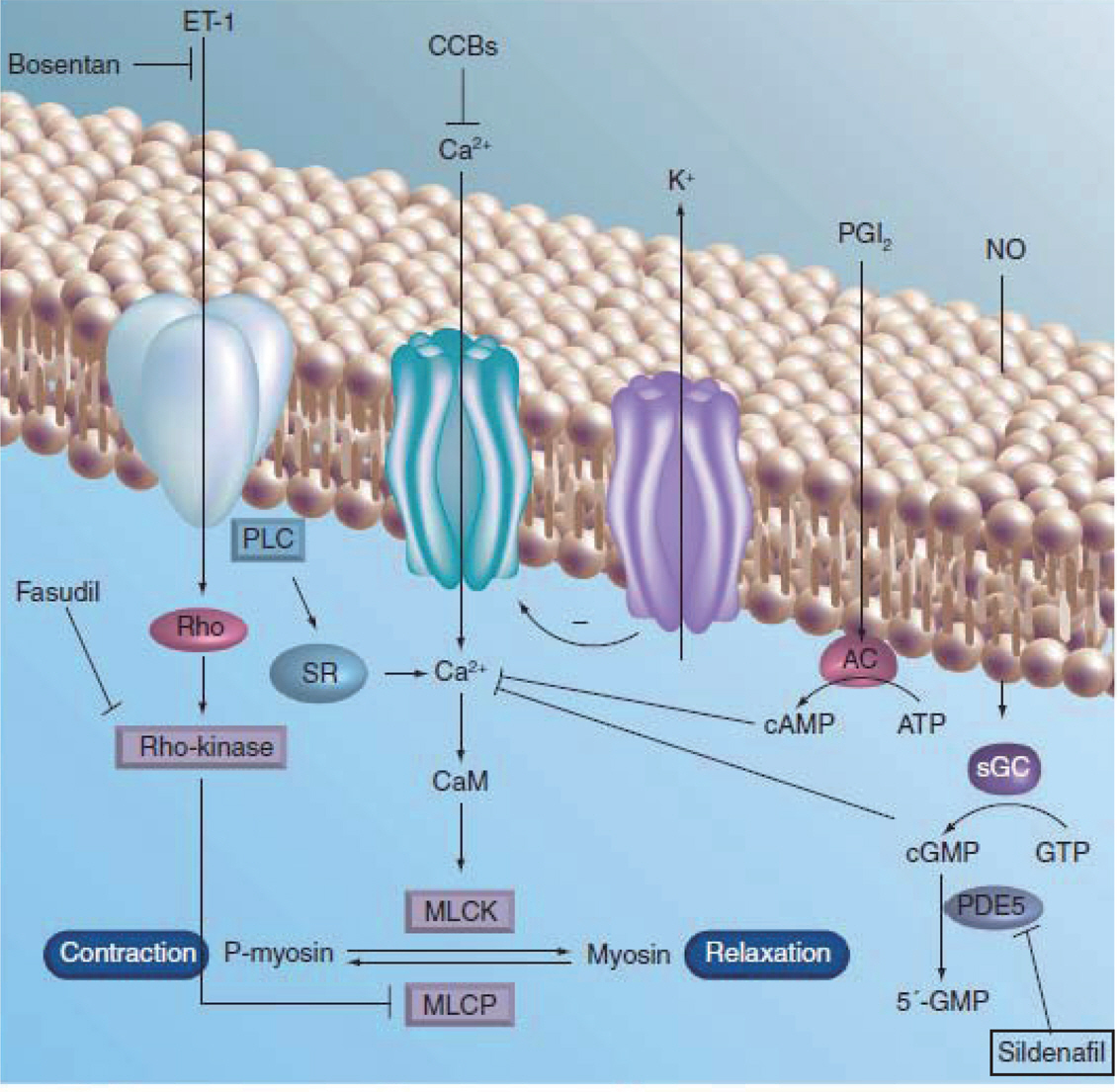
- Neonatal pulmonary hypertension is associated with meconium aspiration syndrome, sepsis, asphyxia, respiratory distress syndrome, congenital diaphragmatic hernia, congenital heart disease, or bronchopulmonary dysplasia. Newborns with pulmonary hypertension are at risk of death, chronic lung disease, neurodevelopmental disability, and other complications. Because of the diverse pathophysiology of the underlying disease, the diagnostic evaluation and therapeutic approach are important. This article will review the pathophysiologic background and the current therapeutic options for neonatal pulmonary hypertension.
Keyword
MeSH Terms
Figure
Cited by 3 articles
-
Clinical Characteristics of Meconium Aspiration Syndrome according to Ventilator Care
Ryoung-Kyoung Lim, Mi-hye Bae, Ah-Young Kim, Young-Mi Han, Kyung-Hee Park, Shin-Yun Byun
Korean J Perinatol. 2015;26(2):121-127. doi: 10.14734/kjp.2015.26.2.121.Risk Factors of Persistent Pulmonary Hypertension of the Newborn in Neonates with Respiratory Diseases
Hyo Hyeon Cha, Sung Yoon Kim, Mi Ra Park, Hye Sun Yoon
Korean J Perinatol. 2015;26(4):312-320. doi: 10.14734/kjp.2015.26.4.312.Pathophysiology and Risk Factors of Pulmonary Hypertension in Infants with Bronchopulmonary Dysplasia
Do-Hyun Kim
Korean J Perinatol. 2014;25(1):1-8. doi: 10.14734/kjp.2014.25.1.1.
Reference
-
1). Walsh-Sukys MC., Tyson JE., Wright LL., Bauer CR., Korones SB., Stevenson DK, et al. Persistent pulmonary hypertension of the newborn in the era before nitric oxide: Practice variation and outcome. Pediatrics. 2000. 105:14–20.2). Rao S., Bartle D., Patole S. Current and future therapeutic options for persistent pulmonary hypertension in the newborn. Expert Rev Cardiovasc Ther. 2010. 8:845–62.
Article3). Rudolph AM. High pulmonary vacular resistance after birth: I. Pathophysiologic considerations and etiologic classification. Clin Pediatr (Phila). 1980. 19:585–90.4). Levin DL., Mills LJ., Parkey M., Garriott K., Campbell W. Constriction of the fetal ductus arteriosus after administration of indomethacin to the pregnant ewe. J Pediatr. 1979. 94:647–50.
Article5). Van Marter LJ., Leviton A., Allred EN., Pagano M., Sullivan KF., Cohen A, et al. Persistent pulmonary hypertension of the newborn and smoking and aspirin and nonsteroidal antiin-flammtory drug consumption during pregnancy. Pediatrics. 1996. 97:658–663.6). Chambers CD., Hermandez-Diaz S., Van Marter LJ., Werler MM., Louik C., Jones KL, et al. Selective serotonin-reuptake inhibitors and risk of persistent pulmonary hypertension of the newborn. N Engl J Med. 2006. 354:579–87.
Article7). Van Marter LJ., Hermandez-Diaz S., Werler MM., Louik C., Mitchell AA. Nonsteroidal anti-inflammatory drugs in late pregnancy and persistent pulmonary hypertension of the newborm. Pediatrics. 2013. 131:79–87.8). Galbally M., Gentile S., Lewis AJ. Further findings linking SSRIs during pregnancy and persistent pulmonary hypertension of the newborn: clinical implications. CNS Drugs. 2012. 26:813–22.9). Dhillon R. The management of neonatal pulmonary hypertension. Arch Dis Child Fetal Neonatal Ed. 2012. 97:F223–8.
Article10). Kovacs G., Berghold A., Scheidl S., Olschewski H. Pulmonary arterial pressure during rest and exercise in healthy subjects: a systematic review. Eur Respir J. 2009. 34:888–94.
Article11). Shah PS., Ohlsson A. Sildenafil of pulmonary hypertension in neonates. Cochrane Database Syst Rev. 2011. 10:CD005494.12). Adatia I. Recent advances in pulmonary vascular disease. Curr Opin Pediatr. 2002. 14:292–7.
Article13). Barman SA., Zhu S., White RE. RhoA/Rho-kinase signaling: a therapeutic target in pulmonary hypertension. Vasc Health Risk Manag. 2009. 5:663–71.
Article14). Mugford M., Elbourne D., Field D. Extracorporeal membrane oxygenation for severe respiratory failure in newborn infants. Cochrane Database Syst Rev. 2008. 16:CD001340.
Article15). Moore C., Tymvios C., Emerson M. Functional regurgitation of vascular and platelet activity during thrombosis by nitric oxide and endothelial nitric oxide synthase. Thromb Haemost. 2010. 104:342–9.16). Bin-Nun A., Schreiber M. Role of iNO in the modulation of pulmonary vascular resistence. J Perinatol. 2008. 28:S84–92.17). Issa A., Lappalainen U., Kleinmam M., Bry K., Hallman M. Inhaled nitric oxide decrease hyperoxia –induced surfactant abnormality in preterm rabbits. Pediatr Res. 1999. 45:247–54.18). Cotton RB., Sundell HW., Zeldin DC., Morrow JD., Roberts LJ., Hazinski TA, et al. Inhaled nitric oxide attenuated hyperoxic lung injury in lambs. Pediatr Res. 2006. 59:142–6.19). McCurnine DC., Pierce RA., Chang LY., Gibson LL., Osborne-Lawrence S., Yoder BA, et al. Inhaled NO improves early pulmonary function and modifies lung growth and elastin deposition in a baboon model of neonatal chronic lung disease. Am J Physiol Lung Cell Mol Physiol. 2005. 288:450–9.20). Konduri GG., Solimano A., Sokol GM., Singer J., Ehrenkranz RA., Singhal N, et al. A randomized trial of early versus standard inhaled nitric oxide therapy in term and near-term newborn infants with hypoxic respiratory failure. Pediatrics. 2004. 113:559–64.
Article21). Clark RH., Kueser TJ., Walker MW., Southgate WM., Huckaby JL., Perez JA, et al. Low-dose nitric oxide therapy for persistent pulmonary hypertension of the newborn. Clinical Inhaled Nitric Oxide Research Group. N Engl J Med. 2000. 342:469–74.22). Davidson D., Barefield ES., Kattwinkel J., Dudell G., Damask M., Straube R, et al. Inhaled nitric oxide for the early treatment of persistent pulmonary hypertension of the term newborn: a randomized, double-masked, placebo-controlled, dose-response, multicenter study. The I-NO/PPHN Study Group. Pediatrics. 1998. 101:325–34.23). Mehats C., Andersen CB., Filopanti M., Jin SL., Conti M. Cyclic nucleotide phosphodiesterase and their role in endocrine cell signaling. Trends Endocrinol Metab. 2002. 13:29–35.24). Shekerdemian LS. Ravn HB, Penny DJ. Intravenous sildenafil lowers pulmonary vascular resistance in a model of neonatal pulmonary hypertension. Am J Respir Crit Care Med. 2002. 65:1098–1102.25). Ichinose F., Erana-Garcia J., Hromi J., Rveh Y., Jones R., Krim L, et al. Nebulized sildenafil is a selective pulmonary vaso-dialtor in lambs with acute pulmonary hypertension. Crit Care Med. 2001. 29:1000–5.26). Michelakis E., Tymchak W., Lien D., Webster L., Hashimoto K., Archer S, et al. Oral sildenafil is an effective and specific pulmonary vasodilator in patients with pulmonary arterial hypertension. Comparison with inhaled nitric oxide. Circulation. 2002. 5:2398–403.27). Joint Formulary Committee. British National Formulary. 2010-2011 ed. London: British Medical Association and Royal Pharmaceutical Society of Great Britain. 2010. 529.28). Vargas-Origel A., Gomez-Rodriguez G., Aldana-Valemzuela C., Vela-Huerta MM., Alarcon-Santos SB., Amador-Licona N. The use of sildenafil in persistent pulmonary hypertension of the newborn. Am J Perinatol. 2010. 27:225–30.
Article29). Steinhorn RH., Kinsella JP., Pierce C., Butrous G., Dilleen M., Oakes M, et al. Intravenous sildenafil in the treatment of neonates with persistent pulmonary hypertension. J Pediatr. 2009. 155:841–7.
Article30). Baquero H. Soliz A, Neira F, Venegas ME, Sola A. Oral sildenafil in infants with persistent pulmonary hypertension of the newborn: a pilot randomized blinded study. Pediatrics. 2006. 117:1077–83.31). Herrea J., Castillo R., Concha E., Soliz A. Oral sildenafil treatment as an alternative to inhaled NO theraphy for persistent pulmonary hypertension of the newborn. Abstract, Pediatric Academic Societies' annual meeting; 2006 April 29-May 2; San Francisco, CA. Texas: Pediatric Academic Societiety. 2006.32). Soliz A., Concha E., Romero F. Oral sildenafil treatment as therapy for persistent pulmonary hypertension of the newborn: a multicenter randomized trial. Abstract, Pediatric Academic Societies' annual meeting. 2009. May 2-5; Baltimore, Maryland. Texas: Pediatric Academic Societiety, 2009.33). Baquero H. Venegas ME, Lorena V, Fredy N, Augusto S. Neuorological follow-up (12-48 months) to patients treated with oral sildenafil for severe neonatal pulmonary hypertension in NICU where the iNO is not available. Abstract, Pediatric Academic Societies' annual meeting; 2008 May 3-6; Honolulu, Hawaii. Texas: Pediatric Academic Society. 2008.34). Noori S. Friedlich P, Wong P, Garingo A, Seri I. Cardiovascular effects of sildenafil in neonates and infants with congenital diaphragmatic hernia and pulmonary hypertension. Neonatology. 2007. 91:92–100.35). Singh R., Choudhury M., Saxena A., Kapoor PM., Juneja R., Kiran U. Inhaled nitroglycerin versus inhaled milrinone in children with congenital heart disease suffering from pulmonary artery hypertension. J Cardiothorac Vasc Anesth. 2010. 24:797–801.
Article36). McNamara P., Laique F., Muang-In S., Whyte H. Milrinone improves oxygenation in neonates with severe persistent pulmonary hypertension of the newborn. J Crit Care. 2006. 21:217–22.
Article37). Matot I., Gozal Y. Pulmonary responses to selective phosphodiesterase-5 and phophodiesterase-3 inhibitors. Chest. 2004. 125:644–51.38). Paradisis M., Evans J., Kluskow M., Osborn D. Randomized trial of milrinone versus placebo for prevention of low systemic blood flow in very preterm infants. J Pediatr. 2009. 154:189–95.
Article39). Mubarak KK. A review of prostaglandin analogs in the management of patients with pulmonary arterial hypertension. Respir Med. 2009. 104:9–21.
Article40). Philips J 3rd., Lyrene R. Prostaglandins, related compounds, and the perinatal pulmonary circulation. Clin Perinatol. 1984. 11:565–79.
Article41). Max M., Rossaint R. Inhaled prostacyclin in the treatment of pulmonary hypertension. Eur J Pediatr. 2002. 141:830–2.
Article42). Eronen M., Pohjavuori M., Andersson S., Pesonen E., Raiviob KO. Prostacyclin treatment for persistent pulmonary hypertension of the newborn. Pediatr Cardiol. 1997. 18:3–7.
Article43). Hougland KT., Null D., Ward RM., Epoprostenol use in PPHN. Abstract, Pediatric Academic Societies' annual meeting; 2005 May 14-17; Washington, DC. Texas: Pediatric Academic Societiety. 2005.44). Mangones T. Intravenous prostacyclin improves oxygenation in PPHN refractory to iNO. Abstract, Pediatric Academic Societies' annual meeting; 2005 May 14-17; Washington, DC. Texas: Pediatric Academic Societiety. 2005.45). Betremieux P., Rolland A., Prioul V., Beuchee A., Pladys P. Systemic hemodynamic effects of the association of inhaled nitric oxide and intravenous prostacyclin for persistent pulmonary hypertension of the newborn. Abstract, Pediatric Academic Societies' annual meeting; 2005 May 14-17; Washington, DC. Texas: Pediatric Academic Societiety. 2005.46). Kelly LK., Porta NF., Goodman DM., Carroll CL., Steinhorn RH. Inhaled prostacyclin for term infants with persistent pulmonary hypertension refractory to inhaled nitric oxide. J Pediatr. 2002. 141:830–2.
Article47). Eifinger F., Sreeram N., Mehler K., Huenseler C., Kribs A., Roth B. Aerosolized iloprost in the treatment of pulmonary hypertension in extremely preterm infants: a pilot study. Klin Padiatr. 2008. 220:66–9.
Article48). De Jaegere A., Van den Ankle J. Endotracheal instillation of prostacyclin in preterm infants with persistent pulmonary hypertension. Eur Respir J. 1998. 12:932–4.
Article49). Hsiao ROS. Inhaled aerosolized prostacyclin (IAP) for premature infants with PPHN. Abstract, Pediatric Academic Societies' annual meeting; 2004 May 1-4; San Francisco, CA. Texas: Pediatric Academic Societiety. 2004.50). Giaid A., Yanagisawa M., Langleben D., Michel RP., Levy R., Shennib H, et al. Expression of endothelin-1 in the lungs of patients with pulmonary hypertension. N Engl J Med. 1993. 328:1732–9.
Article51). Davenport A., Maguire J. Endothelin. Handb Exp Pharmacol. 2006. 295–329.
Article52). Rosenberg AA., Kennaugh J., Koppenhafer SL., Loomis M., Chatfield BA., Abman SH. Elevated immunoreactive endothelin-1 levels in newborn infants with persistent pulmonary hypertension. J Pediatr. 1993. 123:109–14.
Article53). Nakwan N., Choksuchat D., Saksawad R., Thammachote P. Successful treatment of persistent pulmonary hypertension of the newborn with bosentan. Acta Paediatr. 2009. 98:1683–5.
Article54). Goissen C., Ghyselen L., Tourneux P., Krim G., Storme L., Bou P, et al. Persistent pulmonary hypertension of the newborn with transposition of the great arteries: successful treatment with bosentan. Eur J Pediatr. 2008. 167:437–40.
Article55). Lakshminrusimha S., Swartz DD., Gugino SF., Ma CX., Wynn KA., Ryan RM, et al. Oxygen concentration and pul- monary hemodynamics in newborn lambs with pulmonary hypertension. Pediatr Res. 2009. 66:539–44.56). Teng RJ., Eis A., Bakhutashvili I., Arul N., Konduri GG. Increased superoxide production contributes to the impaired angiogenesis of fetal pulmonary arteries with in utero pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2009. 297:184–95.
Article57). Farrow KN., Lakshminrusimha S., Reda WJ., Wedgwood S., Czech L., Gugino SF, et al. Superoxide dismutase restores eNOS expression and function in resistance pulmonary arteries from neonatal lambs with persistent pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2008. 295:979–87.
Article58). Farrow KN., Groh BS., Schumacker PT., Lakshminrusimha S., Czech L., Gugino SF, et al. Hyperoxia increases phosphodiesterase 5 expression and activity in ovine fetal pulmonary artery smooth muscle cells. Circ Res. 2008. 102:226–33.
Article59). Farrow KN., Steinhorn RH. Phosphodiesterases: emerging therapeutic targets for neonatal pulmonary hypertension. Handb Exp Pharmacol. 2011. 204:251–77.
Article60). Robert JD Jr., Fineman JR., Morin FC 3rd., Shaul PW., Rimar S., Schreiber MD, et al. Inhaled nitric oxide and persistent pulmonary hypertension of the newborn. The inhaled nitric oxide study group. N Engl J Med. 1997. 336:605–10.61). Ladha F., Bonnet S., Eaton F., Hashimoto K., Korbutt G., Thebaud D. Sildenafil improves alveolar growth and pulmonary hypertension in hyperoxia-induced lung injury. Am J Respir Crit Care Med. 2005. 172:750–6.
Article62). Su PH., Chen JY. Inhaled nitric oxide in the management of preterm infants with severe respiratory failure. J Perinatol. 2008. 28:112–6.
Article63). de Visser YP., Walther FJ., Laghmani EH., van Wijngaarden s., Nieuwland K., Wagenaar GT. Phosphodiesterase-4 inhibition attenuates pulmonary inflammation in neonatal lung injury. Eur Respir J. 2008. 31:633–44.
Article64). Woyda K., Koebrich S., Reiss I., Rudoloff S., Pullamsetti SS., Rühlmann A, et al. Inhibition of phosphodiesterase 4 enhances lung alveolarisation in neonatal mice exposed to hyperoxia. Eur Respir J. 2009. 33:861–70.
Article65). Essayan DM. Cyclic nucleotide phosphodiesterase (PDE) inhibitors and immunomodulation. Biochem Pharmacol. 1999. 57:965–73.
Article66). U.S. Food and Drug Administration. Revatio (sildenafil): drug safety communication –recommendation against use in children. Silver Spring, MD: U.S. Food and Drug Administration;[updated 2012 Aug 30; accessed 2013 Feb 20]. Available from:. http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm317743.htm.67). Abman SH., Kinsella JP., Rosenzweig EB., Krishnam U., Kulik T., Mullen M, et al. Implications of the U.S. Food and Drug Administration warning against the use of sildenafil for the treatment of pediatric pulmonary hypertension. Am J Respir Crit Care Med. 2013. 187:572–5.68). Ghofrani HA., Wiedemann R., Rose F., Olschewski H., Scher-muly RT., Weissmann N, et al. Combination therapy with oral sildenafil and inhaled iloprost for severe pulmonary hypertension. Ann Intern Med. 2002. 136:515–22.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Indications for Inhaled Nitric Oxide Therapy in Neonates
- Neonatal respiratory distress: recent progress in understanding pathogenesis and treatment outcomes
- Updated clinical classification of pulmonary hypertension
- Clinical Year in Review of Pulmonary Vascular Disease
- Pulmonary Arterial Hypertension