Yonsei Med J.
2012 Nov;53(6):1085-1092. 10.3349/ymj.2012.53.6.1085.
Chronic Stress Induces Neurotrophin-3 in Rat Submandibular Gland
- Affiliations
-
- 1Division of Pathology, Department of Maxillofacial Diagnostic Science, Kanagawa Dental College, Kanagawa, Japan. ktsukino@kdcnet.ac.jp
- 2Research Institute of Salivary Gland and Health Medicine, Kanagawa Dental College, Kanagawa, Japan.
- 3Department of Oral Pathology, Kanagawa Dental College Postgraduate School, Kanagawa, Japan.
- 4Deparment of Pathology, Yokosuka Kyousai Hospital, Kanagawa, Japan.
- 5Division of Orthodontics, Department of Craniofacial Growth and Development Dentistry, Kanagawa Dental College, Kanagawa, Japan.
- KMID: 1414248
- DOI: http://doi.org/10.3349/ymj.2012.53.6.1085
Abstract
- PURPOSE
Plasma neurotrophin-3 (NT-3) levels are associated with several neural disorders. We previously reported that neurotrophins were released from salivary glands following acute immobilization stress. While the salivary glands were the source of plasma neurotrophins in that situation, the association between the expression of neurotrophins and the salivary gland under chronic stress conditions is not well understood. In the present study, we investigated whether NT-3 levels in the salivary gland and plasma were influenced by chronic stress.
MATERIALS AND METHODS
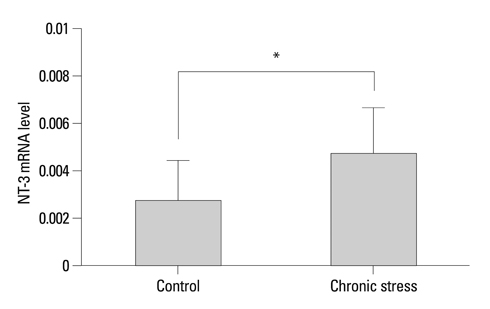
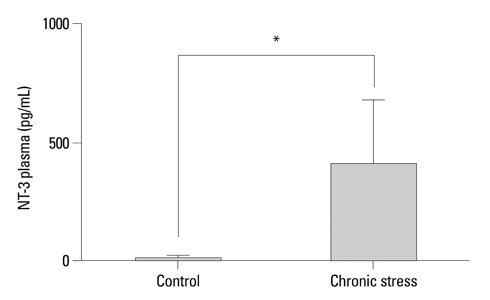
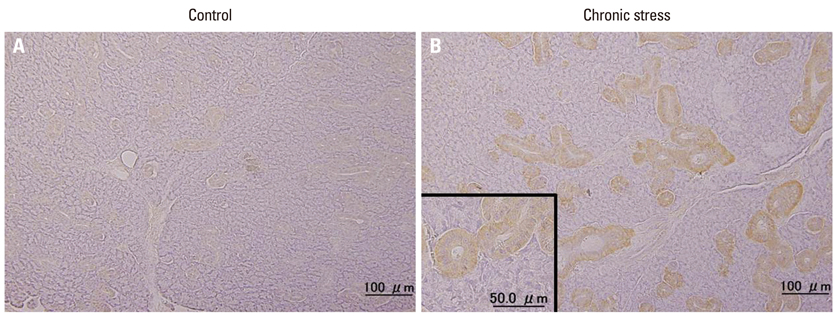
Expressions of NT-3 mRNA and protein were characterized, using real-time polymerase chain reactions, enzyme-linked immunosorbent assay, and immunohistochemistry, in the submandibular glands of male rats exposed to chronic stress (12 h daily for 22 days).
RESULTS
Plasma NT-3 levels were significantly increased by chronic stress (p<0.05), and remained elevated in bilaterally sialoadenectomized rats under the same condition. Since chronic stress increases plasma NT-3 levels in the sialoadenectomized rat model, plasma NT-3 levels were not exclusively dependent on salivary glands.
CONCLUSION
While the salivary gland was identified in our previous study as the source of plasma neurotrophins during acute stress, the exposure to long-term stress likely affects a variety of organs capable of releasing NT-3 into the bloodstream. In addition, the elevation of plasma NT-3 levels may play important roles in homeostasis under stress conditions.
MeSH Terms
Figure
Cited by 1 articles
-
Effects of Stress on Mouse β-Defensin-3 Expression in the Upper Digestive Mucosa
Rie Kawashima, Tomoko Shimizu, Masahiro To, Juri Saruta, Yoshinori Jinbu, Mikio Kusama, Keiichi Tsukinoki
Yonsei Med J. 2014;55(2):387-394. doi: 10.3349/ymj.2014.55.2.387.
Reference
-
1. Mese H, Matsuo R. Salivary secretion, taste and hyposalivation. J Oral Rehabil. 2007. 34:711–723.
Article2. Doel JJ, Hector MP, Amirtham CV, Al-Anzan LA, Benjamin N, Allaker RP. Protective effect of salivary nitrate and microbial nitrate reductase activity against caries. Eur J Oral Sci. 2004. 112:424–428.
Article3. Saruta J, Sato S, Tsukinoki K. The role of neurotrophins related to stress in saliva and salivary glands. Histol Histopathol. 2010. 25:1317–1330.4. Tsukinoki K, Yasuda M, Asano S, Karakida K, Ota Y, Osamura RY, et al. Association of hepatocyte growth factor expression with salivary gland tumor differentiation. Pathol Int. 2003. 53:815–822.
Article5. Cohen S. Purification of a nerve-growth promoting protein from the mouse salivary gland and its neuro-cytotoxic antiserum. Proc Natl Acad Sci U S A. 1960. 46:302–311.
Article6. Cohen S. Isolation of a mouse submaxillary gland protein accelerating incisor eruption and eyelid opening in the new-born animal. J Biol Chem. 1962. 237:1555–1562.
Article7. Aloe L, Alleva E, Böhm A, Levi-Montalcini R. Aggressive behavior induces release of nerve growth factor from mouse salivary gland into the bloodstream. Proc Natl Acad Sci U S A. 1986. 83:6184–6187.
Article8. De Simone R, Alleva E, Tirassa P, Aloe L. Nerve growth factor released into the bloodstream following intraspecific fighting induces mast cell degranulation in adult male mice. Brain Behav Immun. 1990. 4:74–81.
Article9. Hwang DL, Wang S, Chen RC, Lev-Ran A. Trauma, especially of the submandibular glands, causes release of epidermal growth factor into bloodstream in mice. Regul Pept. 1991. 34:133–139.
Article10. Tsukinoki K, Yasuda M, Miyoshi Y, Mori Y, Otsuru M, Saruta J, et al. Role of hepatocyte growth factor and c-Met receptor in neoplastic conditions of salivary glands. Acta Histochem Cytochem. 2005. 38:25–30.
Article11. Lewin GR, Barde YA. Physiology of the neurotrophins. Annu Rev Neurosci. 1996. 19:289–317.
Article12. Poo MM. Neurotrophins as synaptic modulators. Nat Rev Neurosci. 2001. 2:24–32.
Article13. Ernfors P, Ibáñez CF, Ebendal T, Olson L, Persson H. Molecular cloning and neurotrophic activities of a protein with structural similarities to nerve growth factor: developmental and topographical expression in the brain. Proc Natl Acad Sci U S A. 1990. 87:5454–5458.
Article14. Friedman WJ, Ernfors P, Persson H. Transient and persistent expression of NT-3/HDNF mRNA in the rat brain during postnatal development. J Neurosci. 1991. 11:1577–1584.
Article15. Lamballe F, Klein R, Barbacid M. TrkC, a new member of the trk family of tyrosine protein kinases, is a receptor for neurotrophin-3. Cell. 1991. 66:967–979.
Article16. Givalois L, Arancibia S, Alonso G, Tapia-Arancibia L. Expression of brain-derived neurotrophic factor and its receptors in the median eminence cells with sensitivity to stress. Endocrinology. 2004. 145:4737–4747.
Article17. Sharma NK, Ryals JM, Gajewski BJ, Wright DE. Aerobic exercise alters analgesia and neurotrophin-3 synthesis in an animal model of chronic widespread pain. Phys Ther. 2010. 90:714–725.
Article18. Shimazu K, Zhao M, Sakata K, Akbarian S, Bates B, Jaenisch R, et al. NT-3 facilitates hippocampal plasticity and learning and memory by regulating neurogenesis. Learn Mem. 2006. 13:307–315.
Article19. Tazi A, Le Bras S, Lamghitnia HO, Vincent JD, Czernichow P, Scharfmann R. Neurotrophin-3 increases intracellular calcium in a rat insulin-secreting cell line through its action on a functional TrkC receptor. J Biol Chem. 1996. 271:10154–10160.
Article20. Artico M, Bronzetti E, Felici LM, Alicino V, Ionta B, Bronzetti B, et al. Neurotrophins and their receptors in human lingual tonsil: an immunohistochemical analysis. Oncol Rep. 2008. 20:1201–1206.21. Terenghi G. Peripheral nerve regeneration and neurotrophic factors. J Anat. 1999. 194(Pt 1):1–14.
Article22. Tsukinoki K, Saruta J, Sasaguri K, Miyoshi Y, Jinbu Y, Kusama M, et al. Immobilization stress induces BDNF in rat submandibular glands. J Dent Res. 2006. 85:844–848.
Article23. Tsukinoki K, Saruta J, Muto N, Sasaguri K, Sato S, Tan-Ishii N, et al. Submandibular glands contribute to increases in plasma BDNF levels. J Dent Res. 2007. 86:260–264.
Article24. Kuroda Y, McEwen BS. Effect of chronic restraint stress and tianeptine on growth factors, growth-associated protein-43 and microtubule-associated protein 2 mRNA expression in the rat hippocampus. Brain Res Mol Brain Res. 1998. 59:35–39.
Article25. Marais L, van Rensburg SJ, van Zyl JM, Stein DJ, Daniels WM. Maternal separation of rat pups increases the risk of developing depressive-like behavior after subsequent chronic stress by altering corticosterone and neurotrophin levels in the hippocampus. Neurosci Res. 2008. 61:106–112.
Article26. Brondino N, Lanati N, Barale F, Martinelli V, Politi P, Geroldi D, et al. Decreased NT-3 plasma levels and platelet serotonin content in patients with hypochondriasis. J Psychosom Res. 2008. 65:435–439.
Article27. Durany N, Michel T, Zöchling R, Boissl KW, Cruz-Sánchez FF, Riederer P, et al. Brain-derived neurotrophic factor and neurotrophin 3 in schizophrenic psychoses. Schizophr Res. 2001. 52:79–86.
Article28. Mao QQ, Zhong XM, Li ZY, Feng CR, Pan AJ, Huang Z. Herbal formula SYJN increases neurotrophin-3 and nerve growth factor expression in brain regions of rats exposed to chronic unpredictable stress. J Ethnopharmacol. 2010. 131:182–186.
Article29. Walz JC, Frey BN, Andreazza AC, Ceresér KM, Cacilhas AA, Valvassori SS, et al. Effects of lithium and valproate on serum and hippocampal neurotrophin-3 levels in an animal model of mania. J Psychiatr Res. 2008. 42:416–421.
Article30. Nakajima K, Hamada N, Takahashi Y, Sasaguri K, Tsukinoki K, Umemoto T, et al. Restraint stress enhances alveolar bone loss in an experimental rat model. J Periodontal Res. 2006. 41:527–534.
Article31. Lee T, Saruta J, Sasaguri K, Sato S, Tsukinoki K. Allowing animals to bite reverses the effects of immobilization stress on hippocampal neurotrophin expression. Brain Res. 2008. 1195:43–49.
Article32. Smith MA, Makino S, Kvetnansky R, Post RM. Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. J Neurosci. 1995. 15(3 Pt 1):1768–1777.
Article33. Ueyama T, Kawai Y, Nemoto K, Sekimoto M, Toné S, Senba E. Immobilization stress reduced the expression of neurotrophins and their receptors in the rat brain. Neurosci Res. 1997. 28:103–110.
Article34. Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006. 59:1116–1127.
Article35. Takakura AC, Moreira TS, Laitano SC, De Luca LA Júnior, Renzi A, Menani JV. Central muscarinic receptors signal pilocarpine-induced salivation. J Dent Res. 2003. 82:993–997.
Article36. Smith MA. Hippocampal vulnerability to stress and aging: possible role of neurotrophic factors. Behav Brain Res. 1996. 78:25–36.
Article37. Poduslo JF, Curran GL. Permeability at the blood-brain and blood-nerve barriers of the neurotrophic factors: NGF, CNTF, NT-3, BDNF. Brain Res Mol Brain Res. 1996. 36:280–286.
Article38. Hiltunen JO, Arumäe U, Moshnyakov M, Saarma M. Expression of mRNAs for neurotrophins and their receptors in developing rat heart. Circ Res. 1996. 79:930–939.
Article39. Kemi C, Grunewald J, Eklund A, Höglund CO. Differential regulation of neurotrophin expression in human bronchial smooth muscle cells. Respir Res. 2006. 7:18.
Article40. Cassiman D, Denef C, Desmet VJ, Roskams T. Human and rat hepatic stellate cells express neurotrophins and neurotrophin receptors. Hepatology. 2001. 33:148–158.
Article41. Fox EA, McAdams J. Smooth-muscle-specific expression of neurotrophin-3 in mouse embryonic and neonatal gastrointestinal tract. Cell Tissue Res. 2010. 340:267–286.
Article42. Ivy AS, Rodriguez FG, Garcia C, Chen MJ, Russo-Neustadt AA. Noradrenergic and serotonergic blockade inhibits BDNF mRNA activation following exercise and antidepressant. Pharmacol Biochem Behav. 2003. 75:81–88.
Article43. Juric DM, Miklic S, Carman-Krzan M. Monoaminergic neuronal activity up-regulates BDNF synthesis in cultured neonatal rat astrocytes. Brain Res. 2006. 1108:54–62.
Article44. O'Mahony CM, Clarke G, Gibney S, Dinan TG, Cryan JF. Strain differences in the neurochemical response to chronic restraint stress in the rat: relevance to depression. Pharmacol Biochem Behav. 2011. 97:690–699.45. Miklic S, Juric DM, Carman-Krzan M. Differences in the regulation of BDNF and NGF synthesis in cultured neonatal rat astrocytes. Int J Dev Neurosci. 2004. 22:119–130.
Article46. Mattson MP, Maudsley S, Martin B. BDNF and 5-HT: a dynamic duo in age-related neuronal plasticity and neurodegenerative disorders. Trends Neurosci. 2004. 27:589–594.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Localization of Nerves Innervating Sublingual and Submandibular Gland in the CNS Using Cholera Toxin B Subnit and Pseudorabies Virus
- A study of Na+, K+-ATPase in the rat submandibular gland tumor induced by DMBA
- Localization of Nerves Innervating Lacrimal, Submandibular and Sublingual Gland in the Rat Brain Stem Using Cholera Toxin B Subunit
- A Case of Unilateral Absence of the Submandibular Gland Secondary to Sialolithiasis
- Expression and Localization of Epidermal Growth Factor in the Testosterone administered Submandibular Gland of the Rat