Korean J Ophthalmol.
2012 Aug;26(4):241-247. 10.3341/kjo.2012.26.4.241.
Therapeutic Effect of 0.03% Tacrolimus Ointment for Ocular Graft versus Host Disease and Vernal Keratoconjunctivitis
- Affiliations
-
- 1Department of Ophthalmology, Gwangmyeong Sungae Hospital, Gwangmyeong, Korea.
- 2Seoul Balgeun Eye Clinic, Seoul, Korea.
- 3Department of Ophthalmology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. tychung@skku.edu
- KMID: 1387389
- DOI: http://doi.org/10.3341/kjo.2012.26.4.241
Abstract
- PURPOSE
To determine whether topical tacrolimus might prove effective in the treatment of refractory anterior segment inflammatory diseases, and to evaluate its efficacy in eyes with ocular graft versus host disease (GVHD), and vernal keratoconjunctivitis (VKC).
METHODS
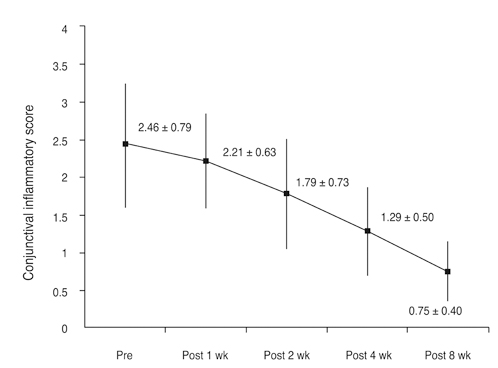
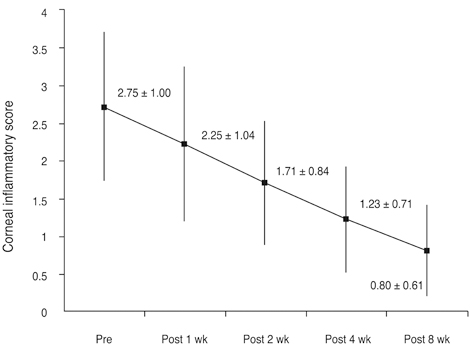
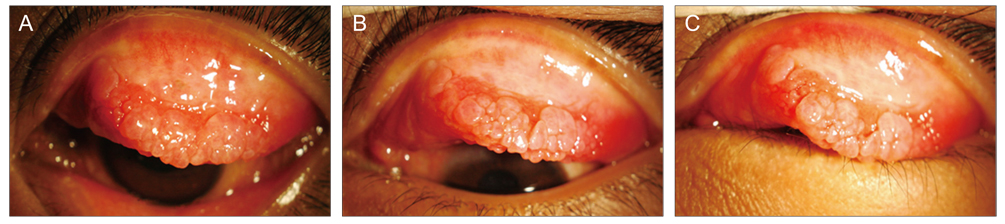
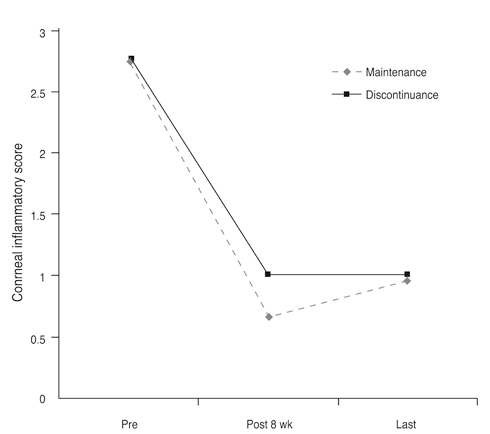
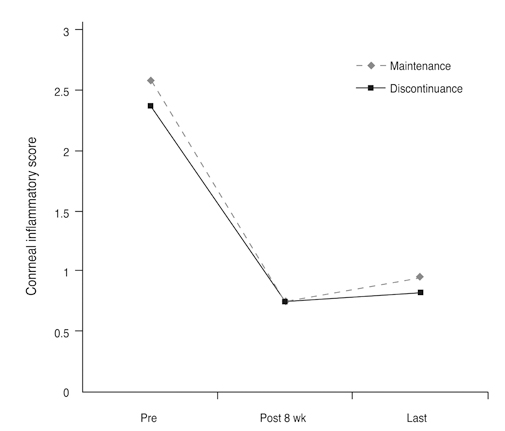
Twenty-eight eyes of 14 patients with anterior segment inflammation refractory to steroid treatment were treated with 0.03% tacrolimus ointment at the Samsung Medical Center, Seoul, Korea from March 2008 through August 2009. Seven patients had ocular GVHD and seven had VKC. We evaluated the conjunctival and corneal inflammatory change at one, two, four, and eight weeks after treatment with a scoring system. Time to initial response of treatment and therapeutic effect between GVHD and VKC was also analyzed. After the eight-week treatment period, patients were divided into two groups (maintenance group and discontinuance group). Eight patients maintained the treatment for an additional four months, and six patients discontinued the treatments. Therapeutic effect was also compared between the groups at eight weeks and six months after treatment.
RESULTS
The mean conjunctival and corneal inflammation score was reduced significantly at eight weeks after treatment (p < 0.0001). The therapeutic effect in conjunctival inflammation was first noted at week two after the initial treatment (p = 0.002); reduction in corneal inflammation was first noted at one week (p = 0.0009). When compared according to diagnosis, no therapeutic difference was detected between the groups (p > 0.05). Six months after treatment, we noted no therapeutic differences between the maintenance group and discontinuance group (p > 0.05).
CONCLUSIONS
0.03% tacrolimus ointment was safe and effective for use in anterior segment inflammatory disease refractory to steroid.
MeSH Terms
Figure
Cited by 1 articles
-
Therapeutic Effects of 0.03% Tacrolimus Eye Drops for Chronic Ocular Graft-Versus-Host Disease
Soon Il Choi, So Hyang Chung
J Korean Ophthalmol Soc. 2015;56(10):1505-1510. doi: 10.3341/jkos.2015.56.10.1505.
Reference
-
1. Frandsen E. Glaucoma and cataract as complications to topical steroid therapy. Acta Ophthalmol (Copenh). 1966. 44:307–312.2. Tanure MA, Cohen EJ, Sudesh S, et al. Spectrum of fungal keratitis at Wills Eye Hospital, Philadelphia, Pennsylvania. Cornea. 2000. 19:307–312.3. Kino T, Hatanaka H, Hashimoto M, et al. FK-506, a novel immunosuppressant isolated from a Streptomyces. I. Fermentation, isolation, and physico-chemical and biological characteristics. J Antibiot (Tokyo). 1987. 40:1249–1255.4. Hooks MA. Tacrolimus, a new immunosuppressant: a review of the literature. Ann Pharmacother. 1994. 28:501–511.5. Knoll GA, Bell RC. Tacrolimus versus cyclosporin for immunosuppression in renal transplantation: meta-analysis of randomised trials. BMJ. 1999. 318:1104–1107.6. Crespo-Leiro MG. Tacrolimus in heart transplantation. Transplant Proc. 2003. 35:1981–1983.7. Bhorade SM, Jordan A, Villanueva J, et al. Comparison of three tacrolimus-based immunosuppressive regimens in lung transplantation. Am J Transplant. 2003. 3:1570–1575.8. Nakagawa H. Comparison of the efficacy and safety of 0.1% tacrolimus ointment with topical corticosteroids in adult patients with atopic dermatitis: review of randomised, double-blind clinical studies conducted in Japan. Clin Drug Investig. 2006. 26:235–246.9. Joseph MA, Kaufman HE, Insler M. Topical tacrolimus ointment for treatment of refractory anterior segment inflammatory disorders. Cornea. 2005. 24:417–420.10. Virtanen HM, Reitamo S, Kari M, Kari O. Effect of 0.03% tacrolimus ointment on conjunctival cytology in patients with severe atopic blepharoconjunctivitis: a retrospective study. Acta Ophthalmol Scand. 2006. 84:693–695.11. Miyazaki D, Tominaga T, Kakimaru-Hasegawa A, et al. Therapeutic effects of tacrolimus ointment for refractory ocular surface inflammatory diseases. Ophthalmology. 2008. 115:988–992.e5.12. Tanaka M, Dogru M, Takano Y, et al. The relation of conjunctival and corneal findings in severe ocular allergies. Cornea. 2004. 23:464–467.13. Martinez-Martinez S, Redondo JM. Inhibitors of the calcineurin/NFAT pathway. Curr Med Chem. 2004. 11:997–1007.14. Schwaninger M, Blume R, Oetjen E, Knepel W. The immunosuppressive drugs cyclosporin A and FK506 inhibit calcineurin phosphatase activity and gene transcription mediated through the cAMP-responsive element in a nonimmune cell line. Naunyn Schmiedebergs Arch Pharmacol. 1993. 348:541–545.15. Glynne R, Akkaraju S, Healy JI, et al. How self-tolerance and the immunosuppressive drug FK506 prevent B-cell mitogenesis. Nature. 2000. 403:672–676.16. Harrison CA, Bastan R, Peirce MJ, et al. Role of calcineurin in the regulation of human lung mast cell and basophil function by cyclosporine and FK506. Br J Pharmacol. 2007. 150:509–518.17. Pleyer U, Lutz S, Jusko WJ, et al. Ocular absorption of topically applied FK506 from liposomal and oil formulations in the rabbit eye. Invest Ophthalmol Vis Sci. 1993. 34:2737–2742.18. Whitcup SM, Pleyer U, Lai JC, et al. Topical liposome-encapsulated FK506 for the treatment of endotoxin-induced uveitis. Ocul Immunol Inflamm. 1998. 6:51–56.19. Dhaliwal JS, Mason BF, Kaufman SC. Long-term use of topical tacrolimus (FK506) in high-risk penetrating keratoplasty. Cornea. 2008. 27:488–493.20. Soter NA, Fleischer AB Jr, Webster GF, et al. Tacrolimus ointment for the treatment of atopic dermatitis in adult patients: part II, safety. J Am Acad Dermatol. 2001. 44:1 Suppl. S39–S46.21. Tuft SJ, Kemeny DM, Dart JK, Buckley RJ. Clinical features of atopic keratoconjunctivitis. Ophthalmology. 1991. 98:150–158.22. Reinhard T, Mayweg S, Reis A, Sundmacher R. Topical FK506 as immunoprophylaxis after allogeneic penetrating normal-risk keratoplasty: a randomized clinical pilot study. Transpl Int. 2005. 18:193–197.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Therapeutic Effects of 0.03% Tacrolimus Eye Drops for Chronic Ocular Graft-Versus-Host Disease
- Therapeutic Effect of Cyclosporine A Eyedrops in Vernal Keratoconjunctivitis
- Acute Cutaneous Graft-Versus-Host Reaction
- Tinea Incognito Caused by Application of 0.03% Tacrolimus (Protopic(R)) Ointment in Atopic Dermatitis Patient
- Diagnosis and pharmacological management of allergic conjunctivitis