Ann Lab Med.
2012 Sep;32(5):331-338. 10.3343/alm.2012.32.5.331.
Clinical Usefulness of Cell-based Indirect Immunofluorescence Assay for the Detection of Aquaporin-4 Antibodies in Neuromyelitis Optica Spectrum Disorder
- Affiliations
-
- 1Department of Laboratory Medicine and Genetics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 2Department of Neurology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. bjkim@skku.edu
- KMID: 1387347
- DOI: http://doi.org/10.3343/alm.2012.32.5.331
Abstract
- BACKGROUND
The presence of antibodies to aquaporin-4 (AQP4) has been identified as a key characteristic of neuromyelitis optica spectrum disorder (NMOSD), an autoimmune inflammatory demyelinating central nervous system (CNS) disorder. We evaluated the performance of a cell-based indirect immunofluorescence assay (CIIFA) for detecting AQP4 antibodies using antigen prepared with a recombinant AQP4 peptide transfection technique and assessed the usefulness of CIIFA for diagnosis of NMOSD in routine clinical practice.
METHODS
Forty-six serum samples from 36 patients as a comparison set and another 101 patients enrolled consecutively from a neurology clinic were included. CIIFA and fluorescence immunoprecipitation assays (FIPA) were performed. CIIFA was performed at 2 different institutions for comparison purposes.
RESULTS
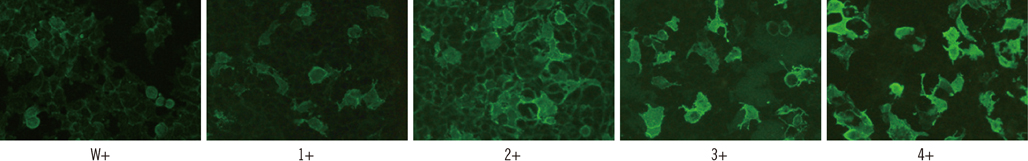
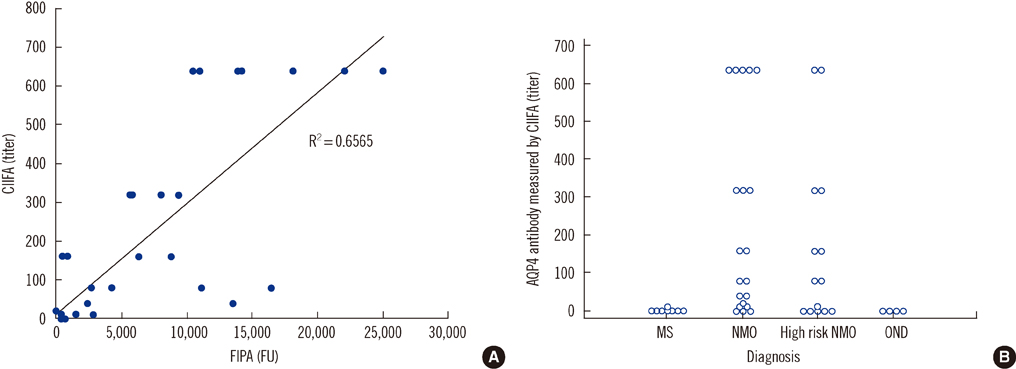
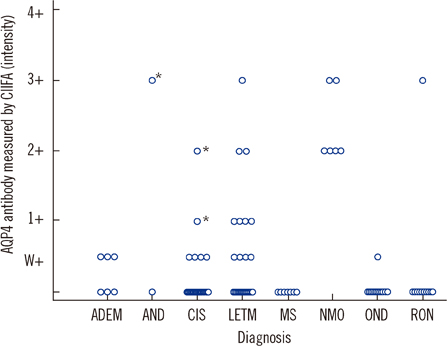
CIIFA and FIPA sensitivity in the comparison set was 86% and 79% in neuromyelitis optica (NMO) patients and 55% and 36% in high-risk NMO patients, respectively. The semiquantitative titer measured by CIIFA correlated well with the arbitrary unit (fluorescence units [FU]) derived from FIPA (r=0.66). Titers measured by CIIFA and FIPA were elevated in NMO patients compared to high-risk NMO patients (1:240 vs. 1:180 and 8,390 vs. 4,059 FU, respectively). The frequency of AQP4 antibody detection by CIIFA in 101 consecutively enrolled patients was 100% in NMO and 23% in high-risk NMO patients, while only 4.6% in control patients, including those with multiple sclerosis.
CONCLUSIONS
Detection of AQP4 antibodies by CIIFA provides sensitive and highly specific diagnostic information for NMO and high-risk NMO patients, which can be used to differentiate these conditions from other demyelinating CNS diseases.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Association of Optic Neuritis with Neuromyelitis Optica Spectrum Disorder and Multiple Sclerosis in Korea
HyoJeong Kim, Kyung-Ah Park, Sei Yeul Oh, Ju-Hong Min, Byoung Joon Kim
Korean J Ophthalmol. 2019;33(1):82-90. doi: 10.3341/kjo.2018.0050.Clinical Usefulness of a Cell-based Assay for Detecting Myelin Oligodendrocyte Glycoprotein Antibodies in Central Nervous System Inflammatory Disorders
Jin Myoung Seok, Patrick Waters, Mi Young Jeon, Hye Lim Lee, Seol-Hee Baek, Jin-Sung Park, Sa-Yoon Kang, Ohyun Kwon, Jeeyoung Oh, Byung-Jo Kim, Kyung-Ah Park, Sei Yeul Oh, Byoung Joon Kim, Ju-Hong Min
Ann Lab Med. 2024;44(1):56-63. doi: 10.3343/alm.2024.44.1.56.
Reference
-
1. Devic E. Subacute myelitis complicated by optic neuritis. Bull Med. 1894. 8:1033–1034.2. Wingerchuk DM, Hogancamp WF, O'Brien PC, Weinshenker BG. The clinical course of neuromyelitis optica (Devic's syndrome). Neurology. 1999. 53:1107–1114.
Article3. de Seze J, Stojkovic T, Ferriby D, Gauvrit JY, Montagne C, Mounier-Vehier F, et al. Devic's neuromyelitis optica: clinical, laboratory, MRI and outcome profile. J Neurol Sci. 2002. 197:57–61.
Article4. Wingerchuk DM, Lennon VA, Lucchinetti CF, Pittock SJ, Weinshenker BG. The spectrum of neuromyelitis optica. Lancet Neurol. 2007. 6:805–815.
Article5. Kira J. Multiple sclerosis in the Japanese population. Lancet Neurol. 2003. 2:117–127.
Article6. Matsushita T, Isobe N, Matsuoka T, Shi N, Kawano Y, Wu XM, et al. Aquaporin-4 autoimmune syndrome and anti-aquaporin-4 antibody-negative opticospinal multiple sclerosis in Japanese. Mult Scler. 2009. 15:834–847.
Article7. Lennon V, Wingerchuk D, Kryzer T, Pittock S, Lucchinetti C, Fujihara K, et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet. 2004. 364:2106–2112.
Article8. Lennon VA. IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J Exp Med. 2005. 202:473–477.
Article9. Hiroaki Y, Tani K, Kamegawa A, Gyobu N, Nishikawa K, Suzuki H, et al. Implications of the aquaporin-4 structure on array formation and cell adhesion. J Mol Biol. 2006. 355:628–639.
Article10. Paul F, Jarius S, Aktas O, Bluthner M, Bauer O, Appelhans H, et al. Antibody to aquaporin 4 in the diagnosis of neuromyelitis optica. PLoS Med. 2007. 4:e133.
Article11. Papeix C, Vidal JS, de Seze J, Pierrot-Deseilligny C, Tourbah A, Stankoff B, et al. Immunosuppressive therapy is more effective than interferon in neuromyelitis optica. Mult Scler. 2007. 13:256–259.
Article12. Cree BA, Lamb S, Morgan K, Chen A, Waubant E, Genain C. An open label study of the effects of rituximab in neuromyelitis optica. Neurology. 2005. 64:1270–1272.
Article13. Keegan M, Pineda AA, McClelland RL, Darby CH, Rodriguez M, Weinshenker BG. Plasma exchange for severe attacks of CNS demyelination: predictors of response. Neurology. 2002. 58:143–146.
Article14. Jarius S, Wildemann B. AQP4 antibodies in neuromyelitis optica: diagnostic and pathogenetic relevance. Nat Rev Neurol. 2010. 6:383–392.
Article15. Waters P, Vincent A. Detection of anti-aquaporin-4 antibodies in neuromyelitis optica: current status of the assays. Int MS J. 2008. 15:99–105.16. Jarius S, Probst C, Borowski K, Franciotta D, Wildemann B, Stoecker W, et al. Standardized methods for the detection of antibodies to aquaporin-4 based on a highly sensitive immunofluorescence assay employing recombinant target antigen. J Neurol Sci. 2010. 291:52–56.17. Hayakawa S, Mori M, Okuta A, Kamegawa A, Fujiyoshi Y, Yoshiyama Y, et al. Neuromyelitis optica and anti-aquaporin-4 antibodies measured by an enzyme-linked immunosorbent assay. J Neuroimmunol. 2008. 196:181–187.
Article18. Takahashi T, Fujihara K, Nakashima I, Misu T, Miyazawa I, Nakamura M, et al. Anti-aquaporin-4 antibody is involved in the pathogenesis of NMO: a study on antibody titre. Brain. 2007. 130:1235–1243.
Article19. Wingerchuk DM, Lennon VA, Pittock SJ, Lucchinetti CF, Weinshenker BG. Revised diagnostic criteria for neuromyelitis optica. Neurology. 2006. 66:1485–1489.
Article20. Waters P, Jarius S, Littleton E, Leite MI, Jacob S, Gray B, et al. Aquaporin-4 antibodies in neuromyelitis optica and longitudinally extensive transverse myelitis. Arch Neurol. 2008. 65:913–919.
Article21. Lucchinetti CF, Mandler RN, McGavern D, Bruck W, Gleich G, Ransohoff RM, et al. A role for humoral mechanisms in the pathogenesis of Devic's neuromyelitis optica. Brain. 2002. 125:1450–1461.22. Takahashi T, Fujihara K, Nakashima I, Misu T, Miyazawa I, Nakamura M, et al. Establishment of a new sensitive assay for anti-human aquaporin-4 antibody in neuromyelitis optica. Tohoku J Exp Med. 2006. 210:307–313.
Article23. Fazio R, Malosio M, Lampasona V, De Feo D, Privitera D, Marnetto F, et al. Antiacquaporin 4 antibodies detection by different techniques in neuromyelitis optica patients. Mult Scler. 2009. 15:1153–1163.
Article24. De Vidi I, Boursier G, Delouche N, Portalès P, Cadars E, Bouthier M, et al. Strategy for anti-aquaporin-4 auto-antibody identification and quantification using a new cell-based assay. Clin Immunol. 2011. 138:239–246.
Article25. Hinson SR, McKeon A, Fryer JP, Apiwattanakul M, Lennon VA, Pittock SJ. Prediction of neuromyelitis optica attack severity by quantitation of complement-mediated injury to aquaporin-4-expressing cells. Arch Neurol. 2009. 66:1164–1167.
Article26. McKeon A, Fryer JP, Apiwattanakul M, Lennon VA, Hinson SR, Kryzer TJ, et al. Diagnosis of neuromyelitis spectrum disorders: comparative sensitivities and specificities of immunohistochemical and immunoprecipitation assays. Arch Neurol. 2009. 66:1134–1138.27. Pittock SJ, Lennon VA, de Seze J, Vermersch P, Homburger HA, Wingerchuk DM, et al. Neuromyelitis optica and non organ-specific autoimmunity. Arch Neurol. 2008. 65:78–83.
Article28. Jarius S, Jacobi C, de Seze J, Zephir H, Paul F, Franciotta D, et al. Frequency and syndrome specificity of antibodies to aquaporin-4 in neurological patients with rheumatic disorders. Mult Scler. 2011. 17:1067–1073.
Article29. Hinson SR, McKeon A, Lennon VA. Neurological autoimmunity targeting aquaporin-4. Neuroscience. 2010. 168:1009–1018.
Article30. Min JH, Kim HJ, Kim BJ, Lee KW, Sunwoo IN, Kim SM, et al. Brain abnormalities in Sjogren syndrome with recurrent CNS manifestations: association with neuromyelitis optica. Mult Scler. 2009. 15:1069–1076.
Article31. Jarius S, Aboul-Enein F, Waters P, Kuenz B, Hauser A, Berger T, et al. Antibody to aquaporin-4 in the long-term course of neuromyelitis optica. Brain. 2008. 131:3072–3080.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Isolated Tongue Paralysis as Presentation of Seropositive Neuromyelitis Optica Spectrum Disorder
- Postpartum Relapse of Neuromyelitis Optica Spectrum Disorder after a Long Period of Spontaneous Remission
- A Case of NMOSD with Double Seropositive for AQP-4 and MOG Antibodies
- Differential Diagnosis between Multiple Sclerosis and Neuromyelitis Optica Spectrum Disorder
- Large-Scale in-House Cell-Based Assay for Evaluating the Serostatus in Patients with Neuromyelitis Optica Spectrum Disorder Based on New Diagnostic Criteria