J Korean Med Sci.
2012 May;27(5):476-483. 10.3346/jkms.2012.27.5.476.
Comparison of Ertapenem and Ceftriaxone Therapy for Acute Pyelonephritis and Other Complicated Urinary Tract Infections in Korean Adults: A Randomized, Double-Blind, Multicenter Trial
- Affiliations
-
- 1Division of Infectious Diseases, Department of Internal Medicine, Korea University College of Medicine, Seoul, Korea. macropha@korea.ac.kr
- 2Division of Infectious Diseases, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 3Department of Internal Medicine, College of Medicine, Inha University, Incheon, Korea.
- 4Division of Infectious Diseases, Department of Internal Medicine, Kangdong Sacred Heart Hospital, Hallym University Medical College, Seoul, Korea.
- 5Department of Internal Medicine, Gachon University, Gil Medical Center, Incheon, Korea.
- 6Department of Infectious Disease, Yonsei University Wonju College of Medicine, Wonju, Korea.
- 7Division of Infectious Diseases, Department of Medicine, Kyung Hee University Medical Center, Kyung Hee University School of Medicine, Seoul, Korea.
- 8Department of Internal Medicine, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea.
- KMID: 1372787
- DOI: http://doi.org/10.3346/jkms.2012.27.5.476
Abstract
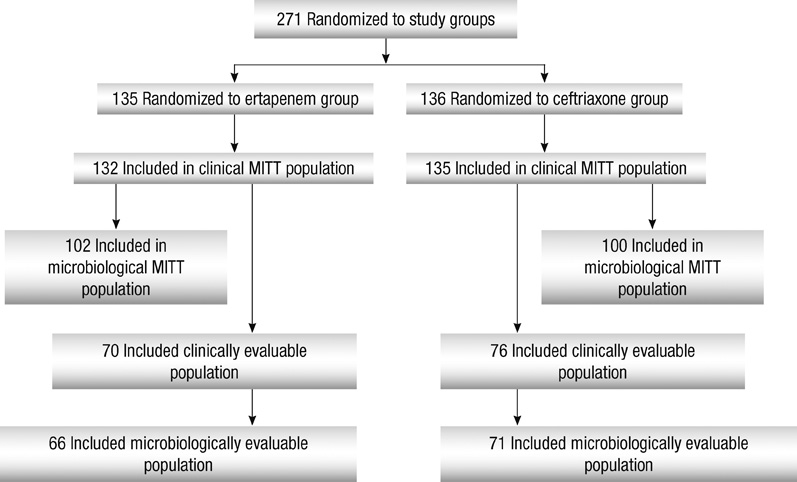
- The efficacy and safety of ertapenem, 1 g once daily, were compared with that of ceftriaxone, 2 g once daily, for the treatment of adults with acute pyelonephritis (APN) and complicated urinary tract infections (cUTIs) in a prospective, multicenter, double-blinded, randomized study. After > or = 3 days of parenteral study therapy, patients could be switched to an oral agent. Of 271 patients who were initially stratified by APN (n = 210) or other cUTIs (n = 61), 66 (48.9%) in the ertapenem group and 71 (52.2%) in the ceftriaxone group were microbiologically evaluable. The mean duration of parenteral and total therapy, respectively, was 5.6 and 13.8 days for ertapenem and 5.8 and 13.8 days for ceftriaxone. The most common pathogen was Escherichia coli. At the primary efficacy endpoint 5-9 days after treatment, 58 (87.9%) patients in the ertapenem group and 63 (88.7%) in the ceftriaxone had a favorable microbiological response. When compared by stratum and severity, the outcomes in the two groups were equivalent. The frequency and severity of drug-related adverse events were generally similar in both treatment groups. The results indicate that ertapenem is highly effective and safe for the treatment of APN and cUTIs.
MeSH Terms
Figure
Cited by 1 articles
-
Comparison of Second- and Third-Generation Cephalosporin as Initial Therapy for Women with Community-Onset Uncomplicated Acute Pyelonephritis
U-Im Chang, Hyung Wook Kim, Seong-Heon Wie
Yonsei Med J. 2015;56(5):1266-1273. doi: 10.3349/ymj.2015.56.5.1266.
Reference
-
1. Lichtenberger P, Hooton TM. Complicated urinary tract infections. Curr Infect Dis Rep. 2008. 10:499–504.2. Ronald AR, Nicolle LE, Stamm E, Krieger J, Warren J, Schaeffer A, Naber KG, Hooton TM, Johnson J, Chambers S, et al. Urinary tract infection in adults: research priorities and strategies. Int J Antimicrob Agents. 2001. 17:343–348.3. Ki M, Park T, Choi B, Foxman B. The epidemiology of acute pyelonephritis in South Korea, 1997-1999. Am J Epidemiol. 2004. 160:985–993.4. Nicolle L. AMMI Canada Guidelines Committee. Complicated urinary tract infection in adults. Can J Infect Dis Med Microbiol. 2005. 16:349–360.5. Warren JW, Abrutyn E, Hebel JR, Johnson JR, Schaeffer AJ, Stamm WE. Guidelines for antimicrobial treatment of uncomplicated acute bacterial cystitis and acute pyelonephritis in women. Infectious Diseases Society of America (IDSA). Clin Infect Dis. 1999. 29:745–758.6. Foxman B, Barlow R, D'Arcy H, Gillespie B, Sobel JD. Urinary tract infection: self-reported incidence and associated costs. Ann Epidemiol. 2000. 10:509–515.7. Naber KG, Bergman B, Bishop MC, Bjerklund-Johansen TE, Botto H, Lobel B, Jinenez Cruz F, Selvaggi FP. Urinary Tract Infection (UTI) Working Group of the Health Care Office (HCO) of the European Association of Urology (EAU). EAU guidelines for the management of urinary and male genital tract infections. Urinary Tract Infection (UTI) Working Group of the Health Care Office (HCO) of the European Association of Urology (EAU). Eur Urol. 2001. 40:576–588.8. Hooton TM, Stamm WE. Diagnosis and treatment of uncomplicated urinary tract infection. Infect Dis Clin North Am. 1997. 11:551–581.9. The Korean Society of Infectious Diseases. The Korean Society for Chemotherapy. Korean Association of Urogenital Tract Infection and Inflammation. The Korean Society of Clinical Microbiology. Clinical guideline for the diagnosis and treatment of urinary tract infections: asymptomatic bacteriuria, uncomplicated & complicated urinary tract infections, bacterial prostatitis. Infect Chemother. 2011. 43:1–25.10. Muratani T, Matsumoto T. Urinary tract infection caused by fluoroquinolone-and cephem-resistant Enterobacteriaceae. Int J Antimicrob Agents. 2006. 28:S10–S13.11. Nicolas-Chanoine MH, Blanco J, Leflon-Guibout V, Demarty R, Alonso MP, Caniça MM, Park YJ, Lavigne JP, Pitout J, Johnson JR. Intercontinental emergence of Escherichia coli clone O25:H4-ST131 producing CTX-M-15. J Antimicrob Chemother. 2008. 61:273–281.12. Cunha BA. Ertapenem. A review of its microbiologic pharmacokinetic and clinical aspects. Drugs Today (Barc). 2002. 38:195–213.13. Solomkin JS, Yellin AE, Rotstein OD, Christou NV, Dellinger EP, Tellado JM, Malafaia O, Fernandez A, Choe KA, Carides A, et al. Ertapenem versus piperacillin/tazobactam in the treatment of complicated intraabdominal infections: results of a double-blind, randomized comparative phase III trial. Ann Surg. 2003. 237:235–245.14. Graham DR, Lucasti C, Malafaia O, Nichols RL, Holtom P, Perez NQ, McAdams A, Woods GL, Ceesay TP, Gesser R. Ertapenem once daily versus piperacillin-tazobactam 4 times per day for treatment of complicated skin and skin-structure infections in adults: results of a prospective, randomized, double-blind multicenter study. Clin Infect Dis. 2002. 34:1460–1468.15. Roy S, Higareda I, Angel-Muller E, Ismail M, Hague C, Adeyi B, Woods GL, Teppler H. Protocol 023 Study Group. Ertapenem once a day versus piperacillin-tazobactam every 6 hours for treatment of acute pelvic infections: a prospective, multicenter, randomized, double-blind study. Infect Dis Obstet Gynecol. 2003. 11:27–37.16. Ortiz-Ruiz G, Caballero-Lopez J, Friedland IR, Woods GL, Carides A. Protocol 018 Ertapenem Community-Acquired Pneumonia Study Group. A study evaluating the efficacy, safety, and tolerability of ertapenem versus ceftriaxone for the treatment of community-acquired pneumonia in adults. Clin Infect Dis. 2002. 34:1076–1083.17. Tomera KM, Burdmann EA, Reyna OG, Jiang Q, Wimmer WM, Woods GL, Gesser RM. Protocol 014 Study Group. Ertapenem versus ceftriaxone followed by appropriate oral therapy for treatment of complicated urinary tract infections in adults: results of a prospective, randomized, double-blind multicenter study. Antimicrob Agents Chemother. 2002. 46:2895–2900.18. Guidance for industry. Complicated urinary tract infections and pyelonephritis-developing antimicrobial durgs for treatment. Center for Drug Evaluation and Research. accessed on 17 September 2010. Available at http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm070981.pdf.19. Wikler MA. Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing: eighteenth informational supplement [M100-S18]. 2008. Wayne, PA: CLSI.20. Jimenez-Cruz F, Jasovich A, Cajigas J, Jiang Q, Imbeault D, Woods GL, Gesser RM. Protocol 021 Study Group. A prospective, multicenter, randomized, double-blind study comparing ertapenem and ceftriaxone followed by appropriate oral therapy for complicated urinary tract infections in adults. Urology. 2002. 60:16–22.21. Wells WG, Woods GL, Jiang Q, Gesser RM. Treatment of complicated urinary tract infection in adults: combined analysis of two randomized, double-blind, multicentre trials comparing ertapenem and ceftriaxone followed by appropriate oral therapy. J Antimicrob Chemother. 2004. 53:ii67–ii74.22. Teppler H, Gesser RM, Friedland IR, Woods GL, Meibohm A, Herman G, Mistry G, Isaacs R. Safety and tolerability of ertapenem. J Antimicrob Chemother. 2004. 53:ii75–ii81.23. Wie SH, Chang UI, Kim HW, Kim YS, Kim SY, Hur J, Kim SI, Kim YR, Kang MW. Clinical features and antimicrobial resistance among clinical isolates of women with community-acquired acute pyelonephritis in 2001-2006. Infect Chemother. 2007. 39:9–16.24. Kim ME, Ha US, Cho YH. Prevalence of antimicrobial resistance among uropathogens causing acute uncomplicated cystitis in female outpatients in South Korea: a multicentre study in 2006. Int J Antimicrob Agents. 2008. 31:S15–S18.25. Ko KS, Lee MY, Song JH, Lee H, Jung DS, Jung SI, Kim SW, Chang HH, Yeom JS, Kim YS, et al. Prevalence and characterization of extended-spectrum beta-lactamase-producing Enterobacteriaceae isolated in Korean hospitals. Diagn Microbiol Infect Dis. 2008. 61:453–459.26. Badal R, Bouchillon S, Hawser S, Hoban D, Hackel M, Hsueh PR. Antimicrobial susceptibility of urinary tract infection pathogens in Asia--SMART 2009. In : 12th Western Pacific Congress on Chemotherapy and Infectious Diseases; 2-5 December 2010; Singapore. Abstract no. P026.27. Alhambra A, Cuadros JA, Cacho J, Gómez-Garcés JL, Alós JI. In vitro susceptibility of recent antibiotic-resistant urinary pathogens to ertapenem and 12 other antibiotics. J Antimicrob Chemother. 2004. 53:1090–1094.28. Livermore DM, Oakton KJ, Carter MW, Warner M. Activity of ertapenem (MK-0826) versus Enterobacteriaceae with potent beta-lactamases. Antimicrob Agents Chemother. 2001. 45:2831–2837.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Randomized, Controlled, Open, Multi-Center Clinical Trial Comparing Ertapenem versus Ceftriaxone plus Metronidazole for the Treatment of Complicated Intra-abdominal Infections in Adults
- Clinical Guideline for the Diagnosis and Treatment of Urinary Tract Infections: Asymptomatic Bacteriuria, Uncomplicated & Complicated Urinary Tract Infections, Bacterial Prostatitis
- A Case of Acute Renal Failure due to Bilateral Acute Pyelonephritis
- Clinical, Bacteriological and Immunololgical Studies of Nonspecific Urinary Tract Infections
- Two Cases of Xanthogranulomatous Pyelonephritis