J Vet Sci.
2012 Mar;13(1):67-71. 10.4142/jvs.2012.13.1.67.
Effect of oxytocin infusion on luteal blood flow and progesterone secretion in dairy cattle
- Affiliations
-
- 1Clinic of Farm Animals, Faculty of Veterinary Medicine, Aristotle University of Thessaloniki, Thessaloniki 54627, Greece. tsousis@vet.auth.gr
- 2Clinic for Cattle, University of Veterinary Medicine, Hanover 30173, Germany.
- 3Department of Obstetrics, Gynaecology and Reproduction, Faculty of Veterinary Medicine, University of Kafkas, Kars 36300, Turkey.
- 4Department of Reproduction, Faculty of Veterinary Medicine and Zootechnics, University of Mexico, Mexico City 04510, Mexico.
- KMID: 1365005
- DOI: http://doi.org/10.4142/jvs.2012.13.1.67
Abstract
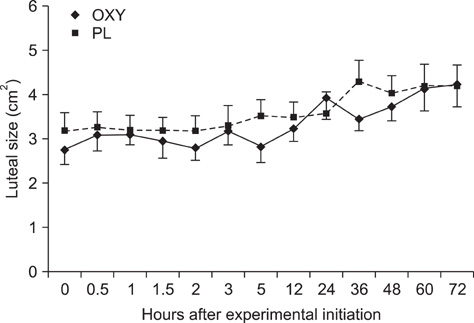
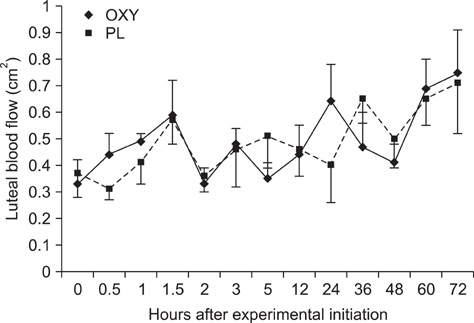
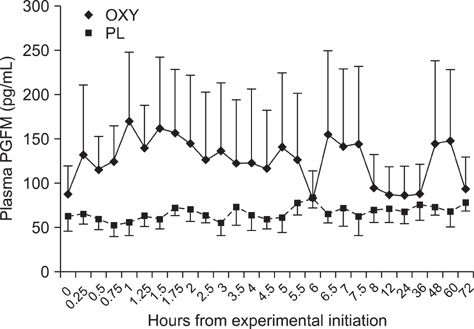
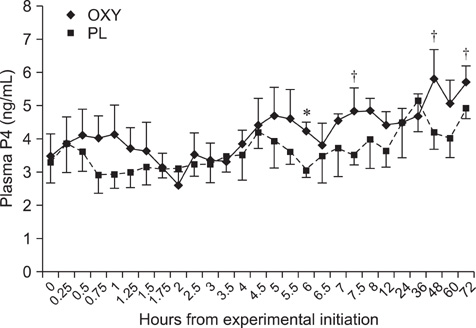
- The objective of this study was to investigate the effects of oxytocin infusion on corpus luteum (CL) function during early to mid-diestrus by measuring luteal size (LS) and luteal blood flow (LBF) along with plasma levels of progesterone (P4) and prostaglandin metabolites (13,14-dihydro-15-keto-prostaglandin F2alpha, PGFM). On day (D) 7 of the estrus cycle (D1 = ovulation), seven cows received 100 IU of oxytocin (OXY) or placebo (PL) following a Latin square design. LS and LBF increased in both groups over time and no differences were observed between the groups. PGFM did not differ either within the groups over time or between the groups at any time point. P4 of the OXY group was higher compared to that of the the PL group 360 min after the infusion (p = 0.01) and tended to be higher at the time points 450 min, 48 h, and 72 h (all p = 0.08). Results from this study support the hypothesis that OXY is not directly involved in the mechanism(s) governing blood flow of the CL and has no remarkable effects either on luteal size or P4 and PGFM plasma levels. Further investigation is needed to elucidate the role of OXY in CL blood flow during early and late luteal phases.
Keyword
MeSH Terms
-
Animals
Cattle/*physiology
Corpus Luteum/blood supply/*drug effects/secretion/ultrasonography
Dinoprost/analogs & derivatives/blood
Estrous Cycle/*drug effects/physiology
Female
Immunoenzyme Techniques/veterinary
Organ Size/physiology
Oxytocin/*pharmacology
Progesterone/blood/*secretion
Random Allocation
Ultrasonography, Doppler, Color/veterinary
Figure
Reference
-
1. Acosta TJ, Hayashi KG, Ohtani M, Miyamoto A. Local changes in blood flow within the preovulatory follicle wall and early corpus luteum in cows. Reproduction. 2003. 125:759–767.
Article2. Acosta TJ, Miyamoto A. Vascular control of ovarian function: ovulation, corpus luteum formation and regression. Anim Reprod Sci. 2004. 82-83:127–140.
Article3. Berisha B, Schams D. Ovarian function in ruminants. Domest Anim Endocrinol. 2005. 29:305–317.
Article4. Fields MJ, Fields PA. Morphological characteristics of the bovine corpus luteum during the estrous cycle and pregnancy. Theriogenology. 1996. 45:1295–1325.
Article5. Flint APF, Sheldrick E. Continuous infusion of oxytocin prevents induction of uterine oxytocin receptor and blocks luteal regression in cyclic ewes. J Reprod Fertil. 1985. 75:623–631.
Article6. Gilbert CL, Lamming GE, Parkinson TJ, Flint AP, Wathes DC. Oxytocin infusion from day 10 after oestrus extends the luteal phase in non-pregnant cattle. J Reprod Fertil. 1989. 86:203–210.
Article7. Grazzini E, Guillon G, Mouillac B, Zingg HH. Inhibition of oxytocin receptor function by direct binding of progesterone. Nature. 1998. 392:509–512.
Article8. Herzog K, Brockhan-Ludemann M, Kaske M, Beindorff N, Paul V, Niemann H, Bollwein H. Luteal blood flow is a more appropriate indicator for luteal function during the bovine estrous cycle than luteal size. Theriogenology. 2010. 73:691–697.
Article9. Ivell R, Brackett KH, Fields MJ, Richter D. Ovulation triggers oxytocin gene expression in the bovine ovary. FEBS Lett. 1985. 190:263–267.
Article10. Jaroszewski J, Kotwica J. Reduction of ovarian oxytocin content from early luteal phase does not affect the corpus luteum secretory function in cattle. Reprod Nutr Dev. 1994. 34:175–182.
Article11. Kotwica J, Skarzynski D, Jaroszewski J, Williams GL, Bogacki M. Uterine secretion of prostaglandin F2alpha stimulated by different doses of oxytocin and released spontaneously during luteolysis in cattle. Reprod Nutr Dev. 1998. 38:217–226.
Article12. McCracken JA, Custer EE, Lamsa JC. Luteolysis: a neuroendocrine-mediated event. Physiol Rev. 1999. 79:263–323.
Article13. Meidan R, Milvae R, Weiss S, Levy N, Friedman A. Intraovarian regulation of luteolysis. J Reprod Fertil Suppl. 1999. 54:217–228.
Article14. Mishra DP, Meyer HHD, Prakash BS. Validation of a sensitive enzymeimmunoassay for 13,14-dihydro-15-keto-PGF2α in buffalo plasma and its application for reproductive health status monitoring. Anim Reprod Sci. 2003. 78:33–46.
Article15. Miyamoto A, Schams D. Oxytocin stimulates progesterone release from microdialyzed bovine corpus luteum in vitro. Biol Reprod. 1991. 44:1163–1170.
Article16. Miyamoto A, Shirasuna K, Hayashi KG, Kamada D, Awashima C, Kaneko E, Acosta TJ, Matsui M. A potential use of color ultrasound as a tool for reproductive management: New observations using color ultrasound scanning that were not possible with imaging only in black and white. J Reprod Dev. 2006. 52:153–160.
Article17. Niswender GD, Juengel JL, Silva PJ, Rollyson MK, McIntush EW. Mechanisms controlling the function and life span of the corpus luteum. Physiol Rev. 2000. 80:1–29.
Article18. Prakash BS, Meyer HH, Schallenberger E, van de Wiel DF. Development of a sensitive enzymeimmunoassay (EIA) for progesterone determination in unextracted bovine plasma using the second antibody technique. J Steroid Biochem. 1987. 28:623–627.
Article19. Pursley JR, Mee MO, Wiltbank MC. Synchronization of ovulation in dairy cows using PGF2α and GnRH. Theriogenology. 1995. 44:915–923.
Article20. Redmer DA, Reynolds LP. Angiogenesis in the ovary. Rev Reprod. 1996. 1:182–192.
Article21. Savio JD, Boland MP, Roche JF. Development of dominant follicles and length of ovarian cycles in post-partum dairy cows. J Reprod Fertil. 1990. 88:581–591.
Article22. Schams D, Berisha B. Regulation of corpus luteum function in cattle-an overview. Reprod Domest Anim. 2004. 39:241–251.
Article23. Shirasuna K, Shimizu T, Hayashi KG, Nagai K, Matsui M, Miyamoto A. Positive association, in local release, of luteal oxytocin with endothelin 1 and prostaglandin F2alpha during spontaneous luteolysis in the cow: a possible intermediatory role for luteolytic cascade within the corpus luteum. Biol Reprod. 2007. 76:965–970.
Article24. Silvia WJ, Raw RE. Regulation of pulsatile secretion of prostaglandin F2α from the ovine uterus by ovarian steroids. J Reprod Fertil. 1993. 98:341–347.
Article25. Skarzynski DJ, Bogacki M, Kotwica J. Changes in ovarian oxytocin secretion as an indicator of corpus luteum response to prostaglandin F2α treatment in cattle. Theriogenology. 1997. 48:733–742.
Article26. Skarzynski DJ, Jaroszewski JJ, Okuda K. Luteotropic mechanisms in the bovine corpus luteum: role of oxytocin, prostaglandin F2α, progesterone and noradrenaline. J Reprod Dev. 2001. 47:125–137.
Article27. Tallam SK, Walton JS, Johnson WH. Effects of oxytocin on cloprostenol-induced luteolysis, follicular growth, ovulation and corpus luteum function in heifers. Theriogenology. 2000. 53:963–979.
Article28. Wathes DC, Denning-Kendall PA. Control of synthesis and secretion of ovarian oxytocin in ruminants. J Reprod Fertil Suppl. 1992. 45:39–52.29. Webb R, Woad KJ, Armstrong DG. Corpus luteum (CL) function: local control mechanisms. Domest Anim Endocrinol. 2002. 23:277–285.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Prostaglandins and Oxytocin on the Contractility of Isolated Detrusor Muscle strips in Rabbit
- Roles of Gonadal Steroids on Exocrine Secretion of Isolated Perfused Rat Pancreas
- Physiology of Lactation
- Effect of platelet activating factor on the secretion of progesterone in the rabbit
- Effects of induced endometritis on uterine blood flow in cows as evaluated by transrectal Doppler sonography