J Korean Rheum Assoc.
2008 Dec;15(4):306-316. 10.4078/jkra.2008.15.4.306.
Increased Indoleamine 2,3-Dioxygenase Expressing CD11c+ CD11b+ Dendritic cells in Oral Tolerance to Type II Collagen
- Affiliations
-
- 1The Rheumatism Research Center, Catholic Research Institute of Medical Science, The Catholic University of Korea, Seoul, Korea. ho0919@catholic.ac.kr
- KMID: 1270161
- DOI: http://doi.org/10.4078/jkra.2008.15.4.306
Abstract
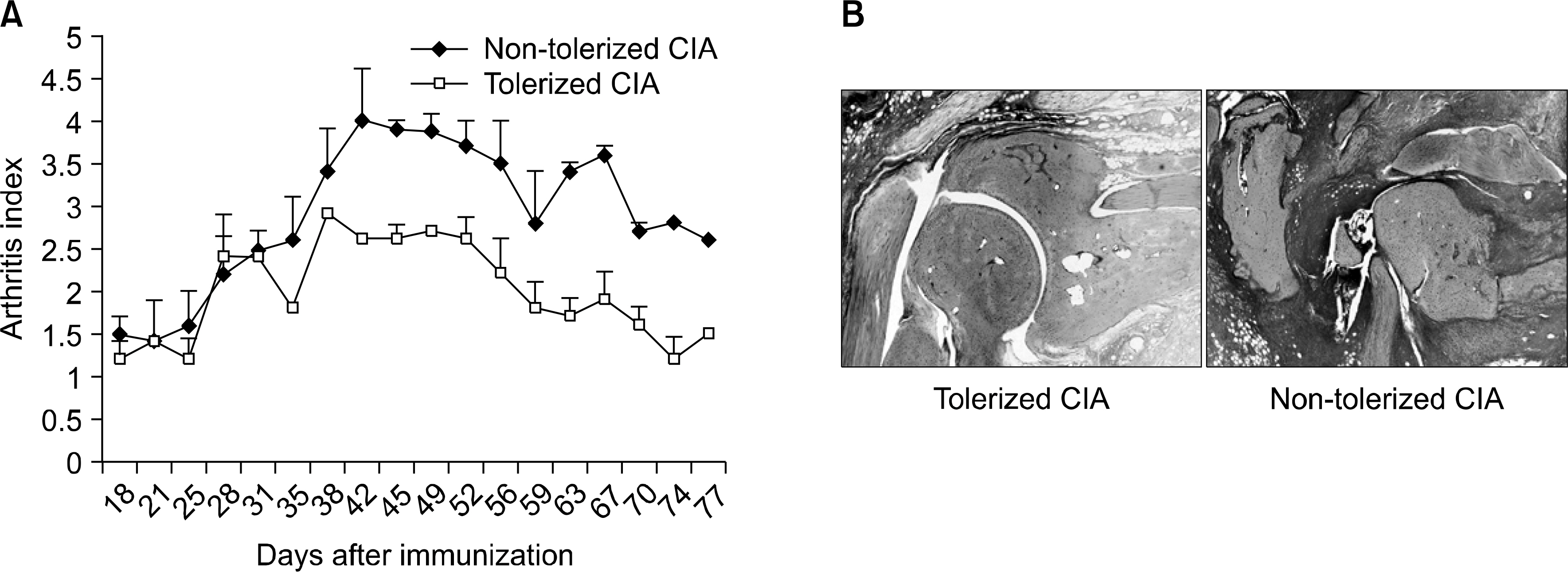
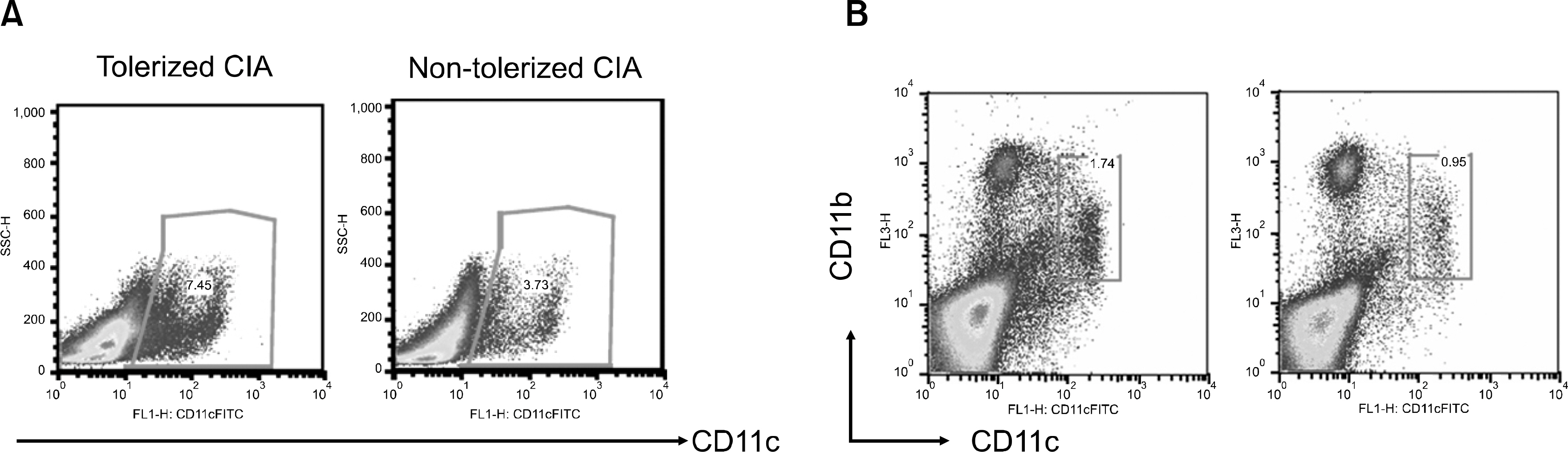
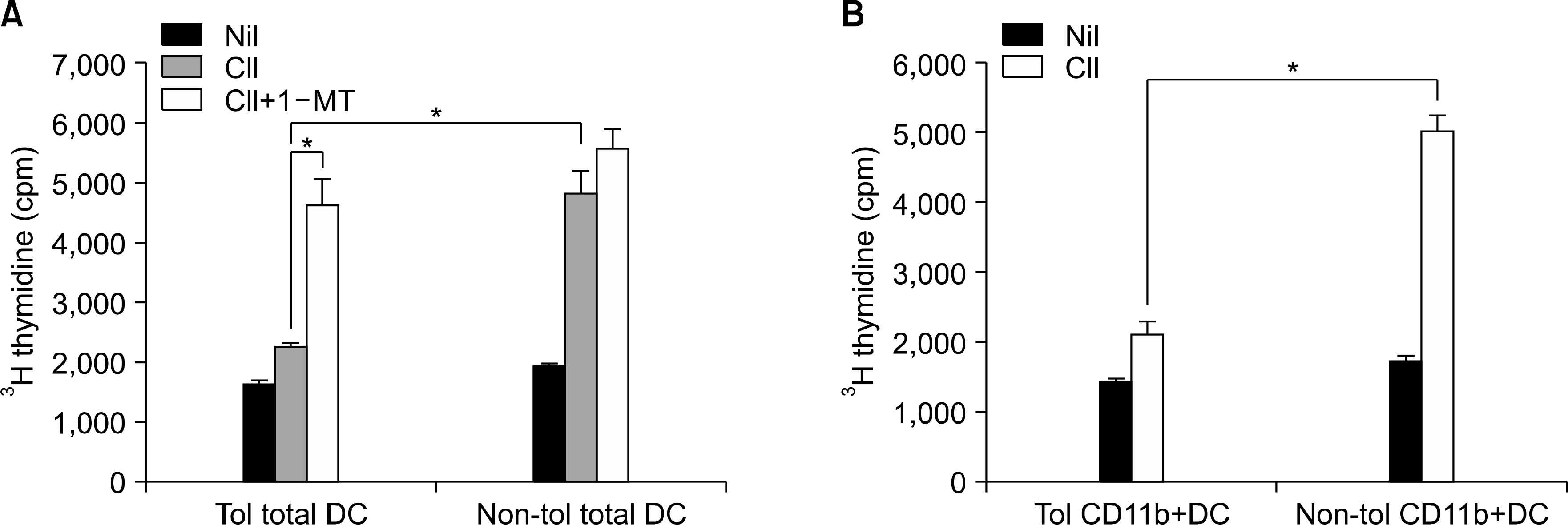
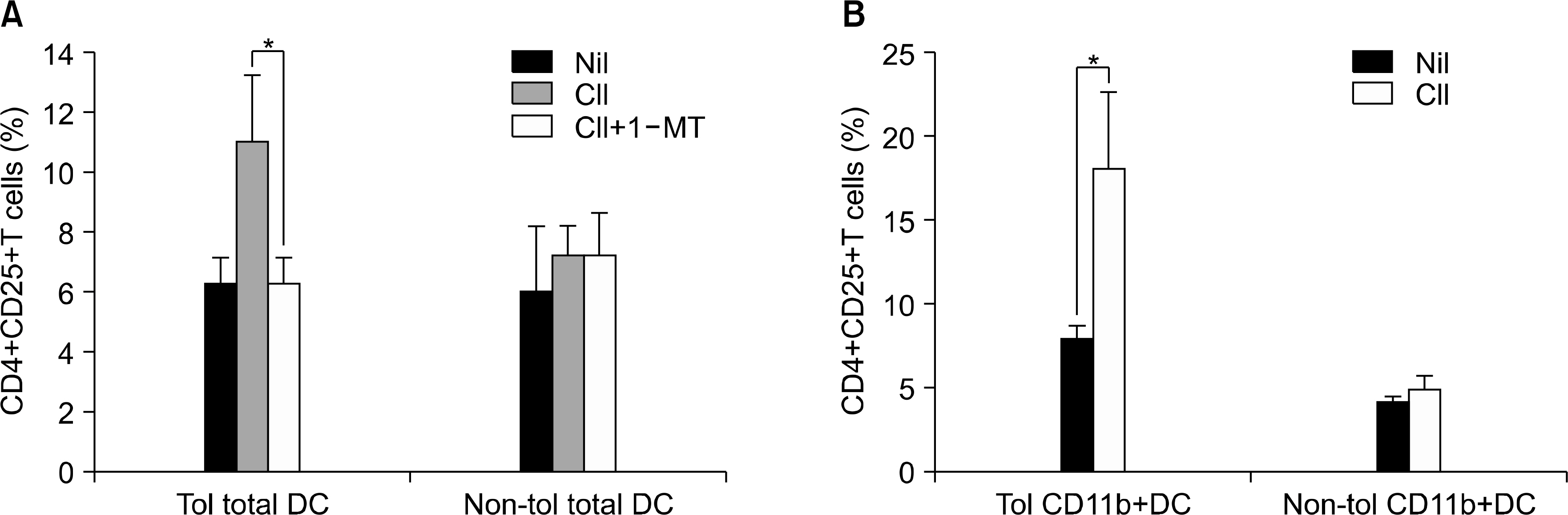
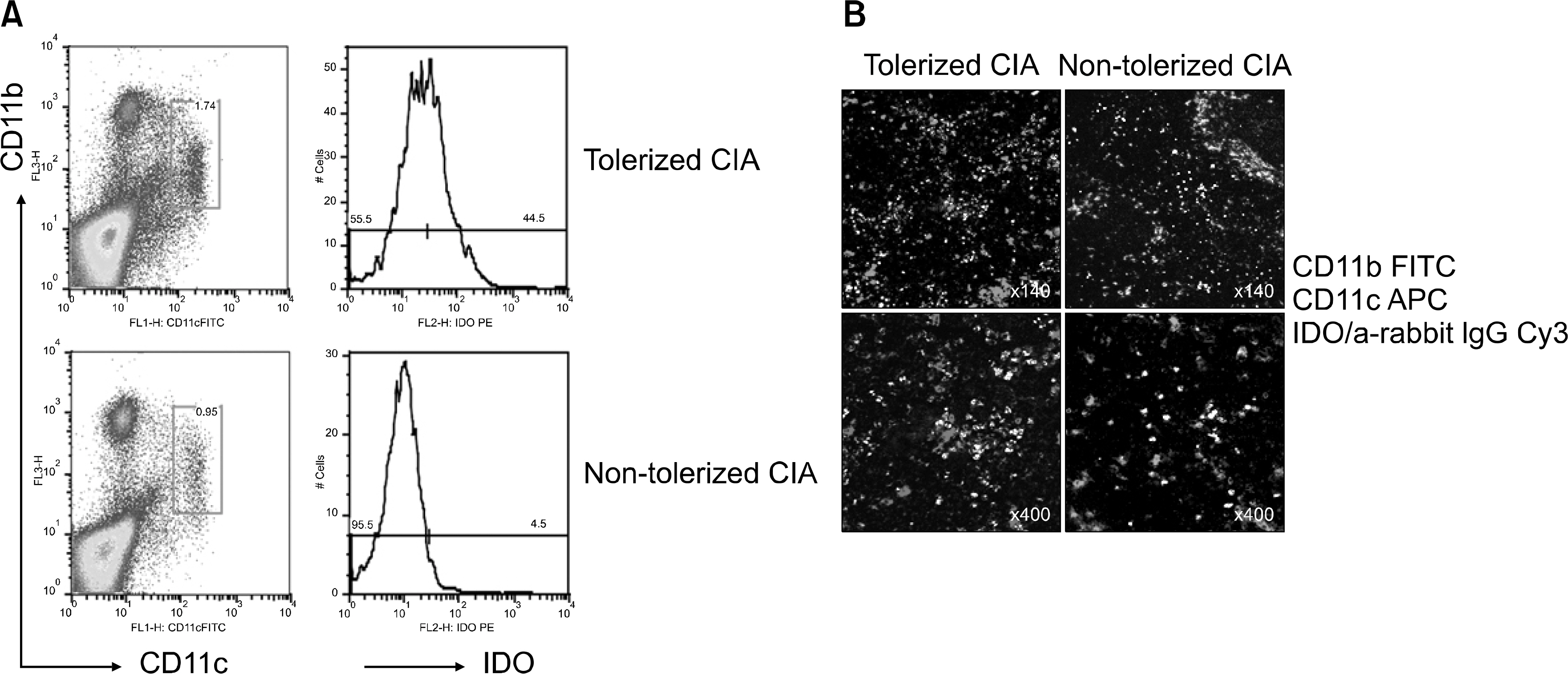
- OBJECTIVE: Indoleamine 2, 3-dioxygenase (IDO), an immuno suppression enzyme, is one of the initial and rate-limiting enzymes involved in the catabolism of the essential amino acid tryptophan. IDO inhibits T cell proliferation, induces T cell apoptosis, and plays a fundamental role in autoimmunity and allergy. We investigated which subtype of dendritic cells (DCs) is involved in IDO expression and the generation of regulatory T cells during the induction of oral tolerance in type II collagen-induced arthritis (CIA). METHODS: Type II Collagen was fed to DBA/1J mice before immunization. Changes in DC subtypes and induction of regulatory T cell in orally tolerized CIA mice were analyzed. Whether the effect of DC subtype was modulated by the IDO expression, was determined by flow cytometry (FACs) and confocal microscopy. RESULTS: IDO expression of CD11c+ DCs was higher in orally tolerized CIA mice than in non-tolerized CIA mice. CD11b+ DCs of the CD11c +DCs, subtype was higher in the induction of in IDO expression. Our data suggest that these IDO expressing DCs of oral tolerized mice suppressed type II collagen-specific T cell proliferation and favored the differentiation of naive CD4+ T cells into regulatory T cells. Especially, CD11c+CD11b+ DCs expressed IDO, which is known to be associated with regulatory T cell induction. CONCLUSION: We observed that oral tolerance induced the increase in IDO-expressing CD11c+CD11b+ DCs, which appeared to induce regulatory T cells. IDO-expressing CD11c+CD11b+ DCs are involved in oral tolerance, which may provide a new therapeutic approach for the treatment of rheumatoid arthritis.
Keyword
Figure
Reference
-
References
1. Nagler-Anderson C, Bober LA, Robinson ME, Siskind GW, Thorbecke GJ. Suppression of type II collageninduced arthritis by intragastric administration of soluble type II collagen. Proc Natl Acad Sci U S A. 1986; 83:7443–6.
Article2. Garcia G, Komagata Y, Slavin AJ, Maron R, Weiner HL. Suppression of collageninduced arthritis by oral or nasal administration of type II collagen. J Autoimmun. 1999; 13:315–24.
Article3. Friedman A, Weiner HL. Induction of anergy or active suppression following oral tolerance is determined by antigen dosage. Proc Natl Acad Sci U S A. 1994; 91:6688–92.
Article4. Mellor AL, Munn D, Chandler P, Keskin D, Johnson T, Marshall B, et al. Tryptophan catabolism and T cell responses. Adv Exp Med Biol. 2003; 527:27–35.
Article5. Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004; 4:762–4.
Article6. Stone TW, Darlington LG. Endogenous kynurenines as targets for drug discovery and development. Nat Rev Drug Discov. 2002; 1:609–20.
Article7. Taylor MW, Feng GS. Relationship between interferon-gamma, indoleamine 2,3-dioxygenase, and tryptophan catabolism. Faseb J. 1991; 5:2516–22.8. Steinman RM, Nussenzweig MC. Avoiding horror autotoxicus: the importance of dendritic cells in peripheral T cell tolerance. Proc Natl Acad Sci U S A. 2002; 99:351–8.
Article9. Derry CJ, Harper N, Davies DH, Murphy JJ, Staines NA. Importance of dose of type II collagen in suppression of collageninduced arthritis by nasal tolerance. Arthritis Rheum. 2001; 44:1917–27.
Article10. Daniels LK: Rapid in-office and in-vivo desensitization of an injection phobia utilizing hypnosis. Am J Clin Hypn. 1976; 18:200–3.11. Mowat AM, Parker LA, Beacock-Sharp H, Millington OR, Chirdo F. Oral tolerance: overview and historical perspectives. Ann N Y Acad Sci. 2004; 1029:1–8.
Article12. Dubois B, Goubier A, Joubert G, Kaiserlian D. Oral tolerance and regulation of mucosal immunity. Cell Mol Life Sci. 2005; 62:1322–32.13. Kelsall BL, Leon F. Involvement of intestinal dendritic cells in oral tolerance, immunity to pathogens, and inflammatory bowel disease. Immunol Rev. 2005; 206:132–48.
Article14. Munn DH, Sharma MD, Lee JR, Jhaver KG, Johnson TS, Keskin DB, et al. Potential regulatory function of human dendritic cells expressing indoleamine 2,3-dioxygenase. Science. 2002; 297:1867–70.
Article15. Grohmann U, Fallarino F, Puccetti P. Tolerance, DCs and tryptophan: much ado about IDO. Trends Immunol. 2003; 24:242–8.
Article16. Min SY, Park KS, Cho ML, Kang JW, Cho YG, Hwang SY, et al. Antigen-induced, tolerogenic CD11c+, CD11b+ dendritic cells are abundant in Peyer's patches during the induction of oral tolerance to type II collagen and suppress experimental collageninduced arthritis. Arthritis Rheum. 2006; 54:887–98.17. Grohmann U, Fallarino F, Silla S, Bianchi R, Belladonna ML, Vacca C, et al. CD40 ligation ablates the tolerogenic potential of lymphoid dendritic cells. J Immunol. 2006; 166:277.
Article18. Mellor AL, Baban B, Chandler PR, Manlapat A, Kahler DJ, Munn DH. Cutting edge: CpG oligonucleotides induce splenic CD19+ dendritic cells to acquire potent indoleamine 2,3-dioxygenasedependent T cell regulatory functions via IFN type 1 signaling. J Immunol. 2005; 175:5601–5.
Article19. Fallarino F, Gizzi S, Mosci P, Grohmann U, Puccetti P. Tryptophan catabolism in IDO+ plasmacytoid dendritic cells. Curr Drug Metab. 2007; 8:209–16.
Article20. Barnett ML, Kremer JM, St Clair EW, Clegg DO, Furst D, Weisman M, et al. Treatment of rheumatoid arthritis with oral type II collagen. Results of a multicenter, double-blind, placebocontrolled trial. Arthritis Rheum. 1998; 41:290–7.
Article21. Park MJ, Min SY, Park KS, Cho YG, Cho ML, Jung YO, et al. Indoleamine 2,3-dioxygenase-expressing dendritic cells are involved in the generation of CD4+CD25+ regulatory T cells in Peyer's patches in an orally tolerized, collageninduced arthritis mouse model. Arthritis Res Ther. 2008; 10:11R.
Article22. Mellor AL, Munn DH. Tryptophan catabolism and T-cell tolerance: immunosuppression by starvation? Immunol Today. 1999; 20:469–73.
Article23. Munn DH, Shafizadeh E, Attwood JT, Bondarev I, Pashine A, Mellor AL. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J Exp Med. 1999; 189:1363–72.
Article24. Bozza S, Fallarino F, Pitzurra L, Zelante T, Montagnoli C, Bellocchio S, et al. A crucial role for tryptophan catabolism at the host/Candida albicans interface. J Immunol. 2005; 174:2910–8.25. Khoury SJ, Hancock WW, Weiner HL. Oral tolerance to myelin basic protein and natural recovery from experimental autoimmune encephalomyelitis are associated with downregulation of inflammatory cytokines and differential upregulation of transforming growth factor β.interleukin 4, and prosta-glandinnE expression in the brain. J Exp Med. 1992; 176:1355–64.26. Weiner HL, Mackin GA, Matusi M, Orav EJ, Khoury SJ, Dawson DM, et al. Double blind pilot trial of oral tolerance with myelin antigen in multiple sclerosis. Science. 1993; 259:1321–4.27. Zhang ZJ, Davidson L, Eisenbarth G, Weiner HL. Suppression of diabetes in nonobese diabetic mice by oral administration of porcine insulin. Proc Natl Acad Sci U S A. 1991; 88:10252–6.
Article28. Nussenblatt RB, Capsi R, Mahdi R, Cahn CC, Roberge F, Lider O, et al. Inhibition of S-antigen induced experimental autoimmune uveoretinitis by oral inducton of tolerance with S-antigen. J Immunol. 1990; 144:1689–94.29. Thompson HSG, Harper N, Bevan DJ, Staines NA. Gastric administration of type II collagen delays the onset and severity of collageninduced arthritis in rats. Clin Exp Immunol. 1986; 6:581–6.30. Mayer L, Shao L. Therapeutic potential of oral tolerance. Nat Rev Immunol. 2004; 4:407–19.
Article31. Hwu P, Du MX, Lapointe R, Do M, Taylor MW, Young HA. Indoleamine 2,3-dioxygenase production by human dendritic cells results in the inhibition of T cell proliferation. J Immunol. 2000; 164:3596–9.
Article32. Mellor AL, Munn DH. Tryptophan catabolism and regulation of adaptive immunity. J Immunol. 2003; 170:5809–13.
Article33. Li H, Zhang GX, Chen Y, Xu H, Fitzgerald DC, Zhao Z, et al. CD11c+CD11b+ dendritic cells play an important role in intravenous tolerance and the suppression of experimental autoimmune encephalomyelitis. J Immunol. 2008; 18:2483–93.
Article34. Chem W, Liang X, Perterson AJ, Muun DH, Blazar BR. The indoleamine 2,3-dioxygenase pathway is essential for human plasmacytoid dendritic cell-induced adaptive T regulatory cell generation. J Immunol. 2008; 181:5396–404.
Article35. Sharma MD, Baban B, Chandler P, Hou DY, Singh N, Yagita H, et al. Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2,3-dioxygenase. J Clin Invest. 2007; 117:2570–82.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immune Regulatory Function of Dendritic Cells Expressing Indoleamine 2,3-Dioxygenase in Orally Tolerance to Type II Collagen-induced Animal Model
- Expression of Local Immunosuppressive Factor, Indoleamine 2,3-dixygenase, in Human Coreal Cells
- The role of placental indoleamine 2,3-dioxygenase in human pregnancy
- Expression of Co-stimulatory Molecules and STAT/SOCS Signaling Factors in the Splenocytes of Mice Tolerized against Arthritis by Oral Administration of Type II Collagen
- Role of Regulatory Cells in Oral Tolerance