J Korean Med Sci.
2007 Aug;22(4):718-721. 10.3346/jkms.2007.22.4.718.
Changes in Serum Adenosine Deaminase Activity during Normal Pregnancy
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Yonsei University College of Medicine, Seoul, Korea. yhkim522@yumc.yonsei.ac.kr
- KMID: 1127093
- DOI: http://doi.org/10.3346/jkms.2007.22.4.718
Abstract
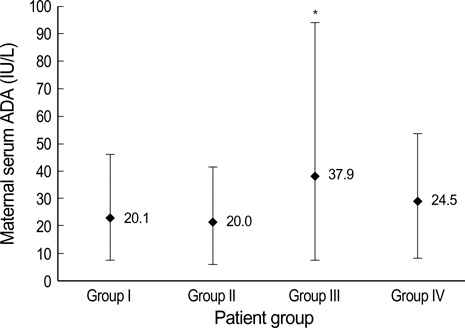
- Adenosine deaminase (ADA), an enzyme essential for the differentiation of lymphoid cells, has been used for monitoring diseases with altered immunity. The purpose of this study was to investigate the changes in serum ADA activity throughout normal pregnancy. We measured the catalytic values of serum ADA from 202 normal pregnant women using a commercial kit. Subjects were divided into four groups according to the gestational age in weeks (Gwks) (Group I: 5-9 Gwks [n=58]; Group II: 15-20 Gwks [n= 63]; Group III: 24-30 Gwks [n=34]; Group IV: 30-39 Gwks [n=47]). The serum ADA levels for the Groups I, II, III, and IV were as follows: 20.1+/-6.9 IU/L, 20.0+/-7.6 IU/L, 37.9+/-19.9 IU/L, and 24.5+/-8.6 IU/L, respectively. The serum ADA activity of group III was significantly higher than the other groups (p<0.05). However, there was no significant correlation between the Gwks and the serum ADA activity. Furthermore, other parameters, such as maternal age (p=0.29), gestational age at delivery (p=0.07), delivery mode (p=0.39), and birth weight (p=0.59) had no correlation with ADA activity. Reference values of serum ADA in normal pregnancy may provide important database for making clinical decisions in pregnancies complicated by conditions where cellular immunity has been altered.
Keyword
MeSH Terms
Figure
Reference
-
1. Martinez-Hernandez D, Arenas-Barbero J, Navarro-Gallar F, Garcia-Esteban R, Santos-Sancho JM, Gomez-de-Terreros FJ. Adenosine deaminase in acquired immunodeficiency syndrome [letter]. Clin Chem. 1988. 34:1949.2. Hitoglou S, Hatzistilianou M, Gougoustamou D, Athanassiadou F, Kotsis A, Catriu D. Adenosine deaminase activity and its isoenzyme pattern in patients with juvenile rheumatoid arthritis and systemic lupus erythematosus. Clin Rheumatol. 2001. 20:411–416.
Article3. Moon J, Han C, Kang S, Park M, Hwang S, Byun M, Chung W, Hwang H, Kim Y, Kim S, Chang J, Kim S. The relationship between age and pleural fluid adenosine deaminase activity in pleural tuberculosis. Tuberc Respir Dis. 2005. 58:459–464.
Article4. Gakis C. Adenosine deaminase (ADA) isoenzymes ADA1 and ADA 2: diagnostic and biological role. Eur Respir J. 1996. 9:632–633.5. Hirschhorn R, Ratech H. Ratazzi MC, Scandalia JG, Whitt GS, editors. Isoenzymes of adenosine deaminase. Current Topics in Biological and Medical Research. 1980. Vol.1. New York: Alan R Liss;132–157.6. Ungerer JP, Oosthuizen HM, Bissbort SH, Vermmak WJ. Serum adenosine deaminase: isoenzymes and diagnostic application. Clin Chem. 1992. 38:1322–1326.
Article7. Adams A, Harkness RA. Adenosine deaminase activity in thymus and other human tissues. Clin Exp Immunol. 1976. 26:647–649.8. Fischer D, Van den Weyden MB, Synderman R, Kelley WN. The role for adenosine deaminase in human monocyte maturation. J Clin Invest. 1976. 58:399–407.9. Galanti S, Nardiello M, Russo F, Fiorentino M. Increased lymphocyte adenosine deaminase in typhoid fever. Scand J Infect Dis. 1981. 13:47–50.
Article10. Saito S, Sakai M, Sasaki Y, Tanabe K, Tsuda H, Michihama T. Quantitative analysis of peripheral blood Th0, Th1, Th2 and the Th1:Th2 cell ratio during normal human pregnancy and preeclampsia. Clin Exp Immunol. 1999. 117:550–555.
Article11. Zuckerman SH, Olson JM, Douglas SD. Adenosine deaminase activity during in vitro culture of human peripheral blood monocytes and pulmonary alveolar macrophages. Exp Cell Res. 1980. 129:281–287.
Article12. Weinberg ED. Pregnancy-associated depression of cell-mediated immunity. Rev Infect Dis. 1984. 6:814–831.
Article13. Rivkees SA, Zhao Z, Porter G, Turner C. Influences of adenosine on the fetus and newborn. Mol Genet Metab. 2001. 74:160–171.
Article14. Henkiewicz J, Michalski J. Adenosine deaminase in pregnancy and in some gynecological diseases. Enzymologia. 1971. 41:261–277.15. Jaqueti J, Martinez-Hernandez D, Hernandez-Garcia R, Navarro-Gallar F, Arenas-Barbero J. Adenosine deaminase in pregnancy serum. Clin Chem. 1990. 36:2144.
Article16. Yoneyama Y, Suzuki S, Sawa R, Otsubo Y, Miura A, Kuwabara Y, Ishino H, Kiyokawa Y, Doi D, Yoneyama K, Araki T. Serum adenosine deaminase activity and its isoenzyme pattern in women with normal pregnancies. Arch Gynecol Obstet. 2003. 267:205–207.
Article17. Hitoglou S, Zournatzi V, Gougoustamou D, Hatzistilianou M, Tzafettas J. Adenosine deaminase activity and its isoenzyme pattern in women with recurrent spontaneous abortions. Gynecol Obstet Invest. 2004. 58:126–129.
Article18. Yoneyama Y, Sawa R, Suzuki S, Otsubo Y, Miura A, Kuwabara Y, Ishino H, Kiyokawa Y, Doi D, Yoneyama K, Kobayashi H, Araki T. Serum adenosine deaminase activity in women with preeclampsia. Gynecol Obstet Invest. 2002. 54:164–167.
Article19. Yoneyama Y, Sawa R, Suzuki S, Otsubo Y, Araki T. Serum adenosine deaminase activity in women with hyperemesis gravidarum. Clin Chim Acta. 2002. 324:141–145.
Article20. Kutlar I, Aksoy F, Koyluoglu O, Ugur MG, Balat O, Tarakcioglu M. Adenosine deaminase activity in serum and placenta of patients with anembryonic pregnancies and missed abortions. Arch Gynecol Obstet. 2005. 272:124–126.
Article21. Gakis C, Naitana A, Ortu AR, Contu A, Bechere M. Adenosine deaminase activity in the diagnosis of infectious diseases. Infect Med. 1994. 11:219–232.22. Melzig M, Paun I. Modulation of adenosine deaminase activity of endothelial cells by steroids. Pharmazie. 1992. 47:394.23. Yoneyama Y, Sawa R, Suzuki S, Ishino H, Miura A, Kuwabara Y, Kuwajima T, Ito N, Kiyokawa Y, Otsubo Y, Araki T. Regulation of plasma adenosine levels in normal pregnancy. Gynecol Obstet Invest. 2002. 53:71–74.
Article24. Agarwal KC. Imai S, Nakazawa M, editors. Modulation of platelet function by plasma adenosine levels. Role of adenosine and adenosine nucleotides in the biology system. 1991. New York: Elsevier;457–468.25. Dekker GA, Sibai BM. The immunology of preeclampsia. Semin Perinatol. 1999. 23:24–33.
Article26. Karabulut AB, Kafkasli A, Burak F, Gozukara EM. Maternal and fetal plasma adensine deaminase, xanthine oxidase and malondialdehyde levels in pre-eclampsia. Cell Biochem Funct. 2005. 23:279–283.27. van Oppen A, van der Tweel I, Alsbach J, Heethaar R, Bruinse H. A longituidinal study of maternal hemodaynamics during normal pregnancy. Obstet Gynecol. 1996. 88:40–46.28. van Oppen A, Stigter R, Bruinse H. Cardiac output in normal pregnancy: a critical review. Obstet Gynecol. 1996. 87:310–318.29. Moser G, Schrader J, Deussen A. Turnover of adenosine in plasma of human and dog blood. Am J Physiol. 1989. 256:C799–C806.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A study on the diagnostic value of cerebrospinal fluid adenosine deaminase activity in children with tuberculous meningitis
- Synovial Fluid Adenosine Deaminse Activity in the Patients of Rheumatoid Arthritis, Osteoarthritis, Ankylosing Spondylitis, and Gouty Arthritis
- Urine Adenosine Deaminase Activity in Confirmed Urinary Tract Tuberculosis
- Diagnostic value of adenosine deaminase and lysozyme activity in pleural effusion
- Adenosine deaminase activity in bronchoalveolar lavage fluid in patients with pulmonary tuberculosis