Korean J Radiol.
2007 Apr;8(2):120-126. 10.3348/kjr.2007.8.2.120.
Variable CT Findings of Epithelial Origin Ovarian Carcinoma According to the Degree of Histologic Differentiation
- Affiliations
-
- 1Department of Radiology and Research Institute of Radiology, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Korea. rialto@amc.seoul.kr
- KMID: 1126835
- DOI: http://doi.org/10.3348/kjr.2007.8.2.120
Abstract
OBJECTIVE
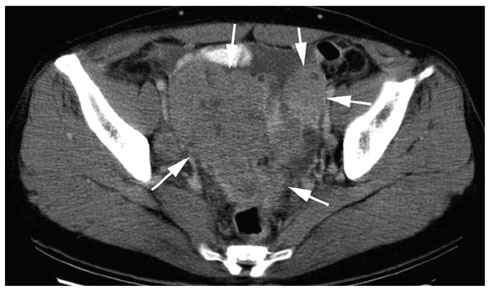
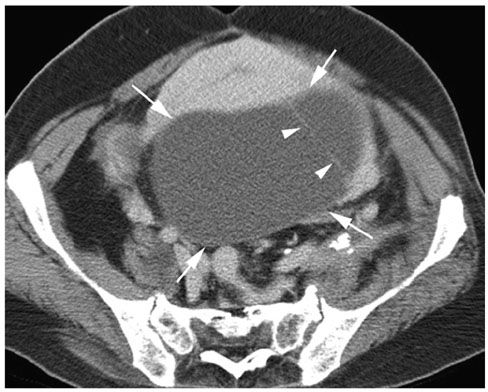
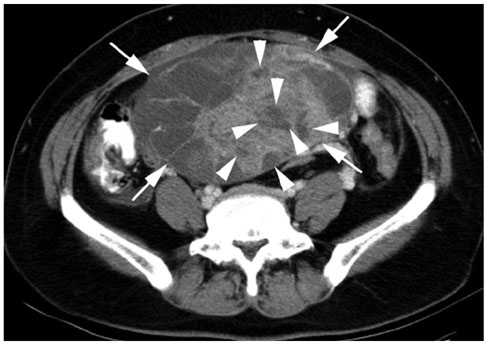
We wanted to evaluate the CT findings of epithelial origin ovarian carcinoma according to the degree of histologic differentiation. MATERIALS AND METHODS: This study enrolled 124 patients with 31 well differentiated, 44 moderately differentiated and 95 poorly differentiated carcinomas with epithelial origin. The CT images were retrospectively evaluated with regard to bilateral ovarian involvement, the tumor's nature, lymphadenopathy, adjacent organ invasion, peritoneal tumor seeding, a large amount of ascites and distant metastasis. In cystic, predominantly cystic and mixed tumors, the tumor wall, septa, papillary projection and necrosis in the solid portion were assessed. RESULTS: Bilateral ovarian involvement was more common in the poorly (48%) and moderately (42%) differentiated carcinomas than in the well differentiated carcinomas (7%) (p < 0.05). The frequency of a predominantly solid or solid nature was greater in the moderately and poorly differentiated carcinomas than in the well differentiated carcinomas (p < 0.0001). In the 87 tumors with a cystic, predominantly cystic or mixed nature, septa greater than 3 mm, papillary projection and necrosis in the solid portion were more common in the poorly differentiated carcinoma (91%, 91% and 77%, respectively) than in the moderately (64%, 68% and 34%, respectively) and well differentiated carcinomas (63%, 47% and 27%, respectively) (p < 0.05). Lymphadenopathy, organ invasion, tumor seeding and a large amount of ascites were more common in the poorly differentiated carcinomas (38%, 27%, 73% and 69%, respectively) than in the moderately (13%, 10%, 48% and 45%, respectively) and well differentiated carcinomas (3%, 0%, 10% and 17%, respectively) (p < 0.05). CONCLUSION: Epithelial origin ovarian carcinoma shows different CT findings according to the degree of histologic differentiation.
MeSH Terms
Figure
Reference
-
1. Landis SH, Murray T, Bolden S, Wingo PA. Cancer statistics 1998. CA Cancer J Clin. 1998. 48:6–29.2. Herrmann UJ Jr, Locher GW, Goldhirsch A. Sonographic patterns of ovarian tumors: prediction of malignancy. Obstet Gynecol. 1987. 69:777–781.3. Ghossain MA, Buy JN, Ligneres C, Bazot M, Hassen K, Malbec L, et al. Epithelial tumors of the ovary: comparison of MR and CT findings. Radiology. 1991. 181:863–870.4. Komatsu T, Konishi I, Mandai M, Togashi K, Kawakami S, Konishi J, et al. Adnexal masses: transvaginal US and gadolinium-enhanced MR imaging assessment of intratumoral structure. Radiology. 1996. 198:109–115.5. Yamashita Y, Hatanaka Y, Torashima M, Takahashi M, Miyazaki K, Okamura H. Characterization of sonographically indeterminate ovarian tumors with MR imaging: a logistic regression analysis. Acta Radiol. 1997. 38:572–577.6. Kurtz AB, Tsimikas JV, Tempany CM, Hamper UM, Arger PH, Bree RL, et al. Diagnosis and staging of ovarian cancer: comparative values of Doppler and conventional US, CT, and MR Imaging correlated with surgery and histopathologic analysis--Report of the Radiology Diagnostic Oncology Group. Radiology. 1999. 212:19–27.7. Kawamoto S, Urban BA, Fishman EK. CT of epithelial ovarian tumors. Radiographics. 1999. 19:S85–S102.8. Jeong YY, Outwater EK, Kang HK. Imaging evaluation of ovarian masses. Radiographics. 2000. 20:1445–1470.9. Hricak H, Chen M, Coakley FV, Kinkel K, Yu KK, Sica G, et al. Complex adnexal masses: detection and characterization with MR imaging-Multivariate analysis. Radiology. 2000. 214:39–46.10. Khoo SK, Battistutta D, Hurst T, Sanderson B, Ward BG, Free K. The prognostic value of clinical, pathologic, and biologic parameters in ovarian cancer. Cancer. 1993. 72:531–537.11. Nascimento AG, Meis-Kindblom JM. Recent advances and controversies in soft tissue pathology. Rev Esp Patol. 1999. 32:424–430.12. Brown DL, Zou KH, Tempany CM, Frates MC, Silverman SG, McNeil BJ, et al. Primary versus secondary ovarian malignancy: imaging findings of adnexal masses in the Radiology Diagnostic Oncology Group Study. Radiology. 2001. 219:213–218.13. Morikawa K, Hatabu H, Togashi K, Kataoka ML, Mori T, Konishi J. Granulosa cell tumor of the ovary: MR findings. J Comput Assist Tomogr. 1997. 21:1001–1004.14. Pretorius ES, Outwater EK, Hunt JL, Siegelman ES. Magnetic resonance imaging of the ovary. Top Magn Reson Imaging. 2001. 12:131–146.15. Barber HR, Sommers SC, Synder R, Kwon TH. Histologic and nuclear grading and stromal reactions as indices for prognosis in ovarian cancer. Am J Obstet Gynecol. 1975. 121:795–807.16. Lieberman MW, Lebovitz RM. Anderson's pathology. 1996. 10th ed. St. Louis: Mosby;513–547.17. Cotran RS, Kumar V, Collins T. Robbins pathologic basis of disease. 1999. 6th ed. Philadelphia: WB Saunder;260–327.18. Giacomarra V, Tirelli G, Papanikolla L, Bussani R. Predictive factors of nodal metastases in oral cavity and oropharynx carcinomas. Laryngoscope. 1999. 109:795–799.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Study on Expression of p53 Protein according to Histologic Types, Degree of Malignancy and Differentiation of the Ovarian Surface Epithelial Tumors
- Expression of c-Met in ovarian epithelial tumor
- Epithelial-Myoepithelial Carcinoma of Intercalated Duct of Parotid Gland
- A Case of Primary Peritoneal Carcinoma
- Two Cases of Clear Cell Carcinoma of Ovary