Clin Orthop Surg.
2009 Jun;1(2):90-95. 10.4055/cios.2009.1.2.90.
Contribution of the Proximal Nerve Stump in End-to-side Nerve Repair: In a Rat Model
- Affiliations
-
- 1Department of Orthopedic Surgery, Yeson Hospital, Bucheon, Korea.
- 2Department of Orthopedic Surgery, Seoul National University College of Medicine, Seoul, Korea. ghbaek@snu.ac.kr
- KMID: 1122638
- DOI: http://doi.org/10.4055/cios.2009.1.2.90
Abstract
-
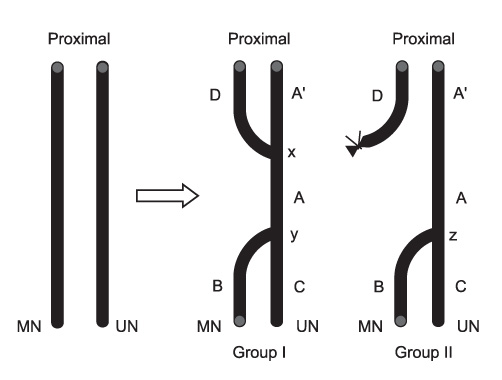
BACKGROUND: The aim of this study was to evaluate the contribution of the proximal nerve stump, in end-to-side nerve repair, to functional recovery, by modifying the classic end-to-side neurorrhaphy and suturing the proximal nerve stump to a donor nerve in a rat model of a severed median nerve.
METHODS
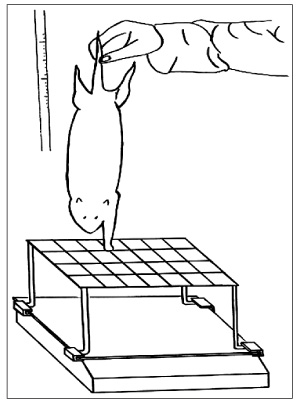
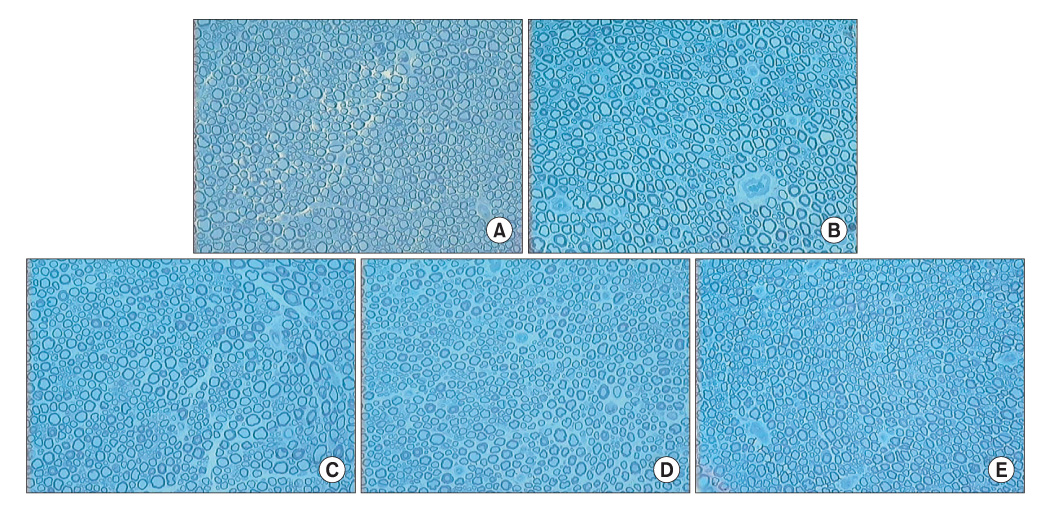
Three experimental groups were studied: a modified end-to-side neurorrhaphy with suturing of the proximal nerve stump (double end-to-side neurorrhaphy, Group I), a classic end-to-side neurorrhaphy (Group II) and a control group without neurorrhaphy (Group III). Twenty weeks after surgery, grasping testing, muscle contractility testing, and histological studies were performed.
RESULTS
The grasping strength, muscle contraction force and nerve fiber count were significantly higher in group I than in group II, and there was no evidence of nerve recovery in group III.
CONCLUSIONS
The contribution from the proximal nerve stump in double end-to-side nerve repair might improve axonal sprouting from the donor nerve and help achieve a better functional recovery in an end-to-side coaptation model.
Keyword
MeSH Terms
Figure
Reference
-
1. Zhao Q, Dahlin LB, Kanje M, Lundborg G. Specificity of muscle reinnervation following repair of the transected sciatic nerve: a comparative study of different repair techniques in the rat. J Hand Surg Br. 1992. 17(3):257–261.
Article2. Millesi H. Peripheral nerve surgery today: turning point or continuous development? J Hand Surg Br. 1990. 15(3):281–287.
Article3. Viterbo F, Trindade JC, Hoshino K, Mazzoni A. Two end-to-side neurorrhaphies and nerve graft with removal of the epineural sheath: experimental study in rats. Br J Plast Surg. 1994. 47(2):75–80.
Article4. Zhao JZ, Chen ZW, Chen TY. Nerve regeneration after terminolateral neurorrhaphy: experimental study in rats. J Reconstr Microsurg. 1997. 13(1):31–37.
Article5. Fortes WM, Noah EM, Liuzzi FJ, Terzis JK. End-to-side neurorrhaphy: evaluation of axonal response and upregulation of IGF-I and IGF-II in a non-injury model. J Reconstr Microsurg. 1999. 15(6):449–457.
Article6. Liu K, Chen LE, Seaber AV, Goldner RV, Urbaniak JR. Motor functional and morphological findings following end-to-side neurorrhaphy in the rat model. J Orthop Res. 1999. 17(2):293–300.
Article7. McCallister WV, Tang P, Smith J, Trumble TE. Axonal regeneration stimulated by the combination of nerve growth factor and ciliary neurotrophic factor in an end-to-side model. J Hand Surg Am. 2001. 26(3):478–488.
Article8. Noah EM, Williams A, Jorgenson C, Skoulis TG, Terzis JK. End-to-side neurorrhaphy: a histologic and morphometric study of axonal sprouting into an end-to-side nerve graft. J Reconstr Microsurg. 1997. 13(2):99–106.
Article9. Bertelli JA, Taleb M, Saadi A, Mira JC, Pecot-Dechavassine M. The rat brachial plexus and its terminal branches: an experimental model for the study of peripheral nerve regeneration. Microsurgery. 1995. 16(2):77–85.
Article10. Bertelli JA, Mira JC. The grasping test: a simple behavioral method for objective quantitative assessment of peripheral nerve regeneration in the rat. J Neurosci Methods. 1995. 58(1-2):151–155.
Article11. Bertelli JA, dos Santos AR, Calixto JB. Is axonal sprouting able to traverse the conjunctival layers of the peripheral nerve? A behavioral, motor, and sensory study of end-to-side nerve anastomosis. J Reconstr Microsurg. 1996. 12(8):559–563.
Article12. Rovak JM, Cederna PS, Kuzon WM Jr. Terminolateral neurorrhaphy: a review of the literature. J Reconstr Microsurg. 2001. 17(8):615–624.
Article13. Yan JG, Matloub HS, Sanger JR, Zhang LL, Riley DA, Jaradeh SS. A modified end-to-side method for peripheral nerve repair: large epineurial window helicoid technique versus small epineurial window standard end-to-side technique. J Hand Surg Am. 2002. 27(3):484–492.
Article14. Karacaoglu E, Yuksel F, Peker F, Guler MM. Nerve regeneration through an epineurial sheath: its functional aspect compared with nerve and vein grafts. Microsurgery. 2001. 21(5):196–201.
Article15. Leibovic SJ, Hastings H 2nd. Martin-Gruber revisited. J Hand Surg Am. 1992. 17(1):47–53.
Article16. Bryan DJ, Wang KK, Chakalis-Haley DP. Effect of Schwann cells in the enhancement of peripheral-nerve regeneration. J Reconstr Microsurg. 1996. 12(7):439–446.
Article17. Bryan DJ, Wang KK, Summerhayes C. Migration of schwann cells in peripheral-nerve regeneration. J Reconstr Microsurg. 1999. 15(8):591–596.
Article18. Alvarez J, Moreno RD, Llanos O, et al. Axonal sprouting induced in the sciatic nerve by the amyloid precursor protein (APP) and other antiproteases. Neurosci Lett. 1992. 144(1-2):130–134.
Article19. Lundborg G, Zhao Q, Kanje M, Danielsen N, Kerns JM. Can sensory and motor collateral sprouting be induced from intact peripheral nerve by end-to-side anastomosis? J Hand Surg Br. 1994. 19(3):277–282.
Article20. McCallister WV, Tang P, Trumble TE. Is end-to-side neurorrhaphy effective? A study of axonal sprouting stimulated from intact nerves. J Reconstr Microsurg. 1999. 15(8):597–603.
Article21. Zhang F, Cheng C, Chin BT, et al. Results of termino-lateral neurorrhaphy to original and adjacent nerves. Microsurgery. 1998. 18(4):276–281.
Article22. Giovanoli P, Koller R, Meuli-Simmen C, et al. Functional and morphometric evaluation of end-to-side neurorrhaphy for muscle reinnervation. Plast Reconstr Surg. 2000. 106(2):383–392.
Article23. Chen YG, Brushart TM. The effect of denervated muscle and Schwann cells on axon collateral sprouting. J Hand Surg Am. 1998. 23(6):1025–1033.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Nerve Regeneration after Modified End-to-Side Neurorrhaphy in the Rat
- Histological and electrophysiological study on nerve regenerationfollowing nerve repair in rat sciatic nerve
- Repetitive Stimulation Test after Sciatic Nerve Section in the Rat
- An experimental study on end-to-side neurorrhaphy in rats
- The Effects of Tension and Immobilization on Nerve Healing in Sutured Peripheral Nerve: An Experimental Study on Rabbit Sciatic Nerve