Korean J Ophthalmol.
2012 Feb;26(1):21-25. 10.3341/kjo.2012.26.1.21.
Clinical Outcomes of Cyclosporine Treatment for Noninfectious Uveitis
- Affiliations
-
- 1Department of Ophthalmology, Jeju National University Hospital, Jeju, Korea.
- 2Department of Ophthalmology, Seoul National University Hospital, Seoul, Korea. hgonyu@snu.ac.kr
- 3Research Institute for Sensory Organs, Medical Research Center, Seoul National University, Seoul, Korea.
- KMID: 1120177
- DOI: http://doi.org/10.3341/kjo.2012.26.1.21
Abstract
- PURPOSE
To assess the clinical outcomes of cyclosporine treatment for noninfectious uveitis.
METHODS
A retrospective review of medical records was completed for 182 noninfectious uveitis patients who were treated with cyclosporine between January 2001 and August 2010. Data was obtained relevant to demographic characteristics, anatomic classification, and laterality of uveitis, associated systemic disorder, dosage of cyclosporine and prednisolone, usage of other immunosuppressive drugs, visual acuity (VA), control of uveitic activity, and adverse effects during the cyclosporine use.
RESULTS
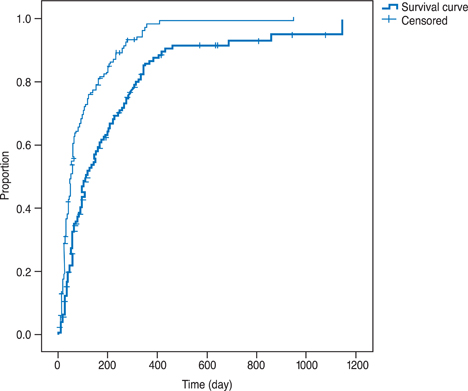
Uveitic activity was controlled to a level of minimal inflammation in 89.0% and completely in 78.6% of patients by the median duration of 49 and 98 days, respectively. Prednisolone-sparing (dose < or =10 mg) control of inflammation equal to or less than the minimal activity was achieved in 75.3% of patients. VA was aggravated more than 0.2 logarithm of the minimum angle of resolution in 17.3% of eyes in spite of cyclosporine treatment for the mean follow-up of 698.4 days. Dose reduction and cessation of cyclosporine was required only in 3.3% and 9.3%, respectively, due to the intolerable toxicity, although 44.0% of patients experienced mild to moderate adverse effects.
CONCLUSIONS
Cyclosporine combined with corticosteroids or other immunosuppressive drugs as needed is an effective treatment for noninfectious uveitis, thus minimizing the adverse effects of corticosteroids and other toxic drugs. However, careful monitoring for the toxicity of cyclosporine is needed, because a small group of patients cannot tolerate its toxicity.
MeSH Terms
-
Adolescent
Adult
Aged
Cyclosporine/administration & dosage/*therapeutic use
Female
Humans
Immunosuppressive Agents/administration & dosage/*therapeutic use
Kaplan-Meier Estimate
Male
Middle Aged
Prednisolone/administration & dosage/therapeutic use
Republic of Korea
Retrospective Studies
Treatment Outcome
Uveitis/*drug therapy
Visual Acuity
Figure
Reference
-
1. Murphy KP, Travers P, Walport M. Murphy KP, Jameway C, Travers P, Walport M, editors. Manipulation of the immune response. Janeway's immunobiology. 2008. 7th ed. New York: Garland Science;659–660.2. Nussenblatt RB, Palestine AG, Chan CC. Cyclosporin A therapy in the treatment of intraocular inflammatory disease resistant to systemic corticosteroids and cytotoxic agents. Am J Ophthalmol. 1983. 96:275–282.3. Calne RY, White DJ, Thiru S, et al. Cyclosporin A in patients receiving renal allografts from cadaver donors. Lancet. 1978. 2:1323–1327.4. Nussenblatt RB, Mittal KK, Ryan S, et al. Birdshot retinochoroidopathy associated with HLA-A29 antigen and immune responsiveness to retinal S-antigen. Am J Ophthalmol. 1982. 94:147–158.5. Nussenblatt RB, Palestine AG, Chan CC. Cyclosporine therapy for uveitis: long-term followup. J Ocul Pharmacol. 1985. 1:369–382.6. Binder AI, Graham EM, Sanders MD, et al. Cyclosporin A in the treatment of severe Behçet's uveitis. Br J Rheumatol. 1987. 26:285–291.7. BenEzra D, Cohen E, Chajek T, et al. Evaluation of conventional therapy versus cyclosporine A in Behçet's syndrome. Transplant Proc. 1988. 20:3 Suppl 4. 136–143.8. Le Hoang P, Girard B, Deray G, et al. Cyclosporine in the treatment of birdshot retinochoroidopathy. Transplant Proc. 1988. 20:3 Suppl 4. 128–130.9. Masuda K, Nakajima A, Urayama A, et al. Double-masked trial of cyclosporin versus colchicine and long-term open study of cyclosporin in Behcet's disease. Lancet. 1989. 1:1093–1096.10. De Vries J, Baarsma GS, Zaal MJ, et al. Cyclosporin in the treatment of severe chronic idiopathic uveitis. Br J Ophthalmol. 1990. 74:344–349.11. Secchi AG, Tognon MS, Maselli C. Cyclosporine-A in the treatment of serpiginous choroiditis. Int Ophthalmol. 1990. 14:395–399.12. Nussenblatt RB, Palestine AG, Chan CC, et al. Randomized, double-masked study of cyclosporine compared to prednisolone in the treatment of endogenous uveitis. Am J Ophthalmol. 1991. 112:138–146.13. Ozyazgan Y, Yurdakul S, Yazici H, et al. Low dose cyclosporin A versus pulsed cyclophosphamide in Behcet's syndrome: a single masked trial. Br J Ophthalmol. 1992. 76:241–243.14. Vitale AT, Rodriguez A, Foster CS. Low-dose cyclosporine therapy in the treatment of birdshot retinochoroidopathy. Ophthalmology. 1994. 101:822–831.15. Araujo AA, Wells AP, Dick AD, Forrester JV. Early treatment with cyclosporin in serpiginous choroidopathy maintains remission and good visual outcome. Br J Ophthalmol. 2000. 84:979–982.16. Michel SS, Ekong A, Baltatzis S, Foster CS. Multifocal choroiditis and panuveitis: immunomodulatory therapy. Ophthalmology. 2002. 109:378–383.17. Ozdal PC, Ortac S, Taskintuna I, Firat E. Long-term therapy with low dose cyclosporin A in ocular Behcet's disease. Doc Ophthalmol. 2002. 105:301–312.18. Becker MD, Wertheim MS, Smith JR, Rosenbaum JT. Long-term follow-up of patients with birdshot retinochoroidopathy treated with systemic immunosuppression. Ocul Immunol Inflamm. 2005. 13:289–293.19. Kiss S, Ahmed M, Letko E, Foster CS. Long-term follow-up of patients with birdshot retinochoroidopathy treated with corticosteroid-sparing systemic immunomodulatory therapy. Ophthalmology. 2005. 112:1066–1071.20. Kacmaz RO, Kempen JH, Newcomb C, et al. Cyclosporine for ocular inflammatory diseases. Ophthalmology. 2010. 117:576–584.21. Mathews D, Mathews J, Jones NP. Low-dose cyclosporine treatment for sight-threatening uveitis: efficacy, toxicity, and tolerance. Indian J Ophthalmol. 2010. 58:55–58.22. Kim HB, Kim EK, Park K, Chung H. Results of cyclotporin A therapy in Behcet's disease. J Korean Ophthalmol Soc. 1990. 31:469–475.23. Lee ET, Kim MH, Chung SM. The effect of cyclosporin therapy on endogenous uveitis. J Korean Ophthalmol Soc. 1997. 38:601–607.24. Jabs DA, Rosenbaum JT, Foster CS, et al. Guidelines for the use of immunosuppressive drugs in patients with ocular inflammatory disorders: recommendations of an expert panel. Am J Ophthalmol. 2000. 130:492–513.25. Jabs DA, Nussenblatt RB, Rosenbaum JT, et al. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005. 140:509–516.26. Tappeiner C, Roesel M, Heinz C, et al. Limited value of cyclosporine A for the treatment of patients with uveitis associated with juvenile idiopathic arthritis. Eye (Lond). 2009. 23:1192–1198.27. Isnard Bagnis C, Tezenas du Montcel S, Beaufils H, et al. Long-term renal effects of low-dose cyclosporine in uveitis-treated patients: follow-up study. J Am Soc Nephrol. 2002. 13:2962–2968.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Fas/FasL Expression in the Anterior Chamber Cells of Patients with Chronic Noninfectious Uveitis
- Effects of Cyclosporin A on the Recurrence in Experimental Autoimmune Anterior Uveitis
- The Effect of Adalimumab in Korean Patients with Refractory Noninfectious Uveitis
- Incidence and Prevalence of Pediatric Noninfectious Uveitis in Korea: A Population-Based Study
- Quiescence and Subsequent Anterior Chamber Inflammation in Adalimumab-treated Pediatric Noninfectious Uveitis