Korean J Radiol.
2010 Dec;11(6):594-602. 10.3348/kjr.2010.11.6.594.
Role of Duplex Power Doppler Ultrasound in Differentiation between Malignant and Benign Thyroid Nodules
- Affiliations
-
- 1Department of Radiology, Ataturk Training and Research Hospital Bilkent, Ankara, Turkey. droktayalgin@gmail.com
- 2Department of Oncology, Gazi University Medical Faculty, Ankara, Turkey.
- 3Department of Radiology, Uludag University Medical Faculty, Gorukle, Bursa, Turkey.
- 4Department of Biostatistics, Uludag University Medical Faculty, Gorukle, Bursa, Turkey.
- 5Department of Pathology, Uludag University Medical Faculty, Gorukle, Bursa, Turkey.
- 6Department of Endocrinology, Uludag University Medical Faculty, Gorukle, Bursa, Turkey.
- KMID: 1119221
- DOI: http://doi.org/10.3348/kjr.2010.11.6.594
Abstract
OBJECTIVE
To evaluate the usage of duplex power Doppler ultrasound (PDUS) for the differentiation of benign and malignant thyroid nodules.
MATERIALS AND METHODS
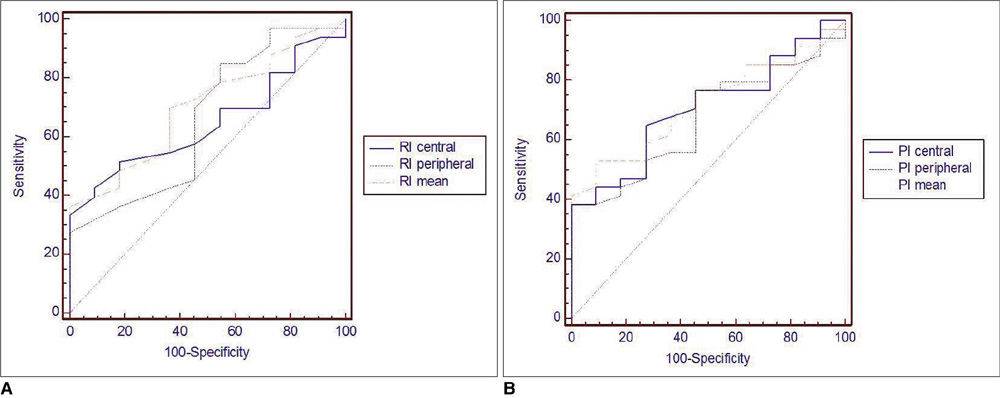
We prospectively examined 77 thyroid nodules in 60 patients undergoing ultrasound-guided fine needle aspiration biopsy (FNAB). Each nodule was described according to size, inner structure, borders, parenchymal echogenicity, peripheral halo formation, and the presence of calcification (B-mode ultrasound findings). Vascularity as determined by PDUS imaging was defined as non-vascular, peripheral, central, or of mixed type. For each nodule, the pulsatility index (PI) and resistive index (RI) values were obtained. Results of FNAB and surgical pathological examination (if available) were used as a proof of final diagnosis to categorize all nodules as benign or malignant. A receiver operating characteristic (ROC) curve analysis was performed to establish cut-off, sensitivity, and specificity values associated with RI-PI values.
RESULTS
A significant relationship was observed between malignancy and irregular margins, microcalcifications, and hypoechogenicity on ultrasound examination (p < 0.05). The pattern of vascularity as determined by PDUS analysis was not a statistically significant criterion to suggest benign or malignant disease in this study (p > 0.05). The central, peripheral, and mean RI-PI values were higher in malignant nodules when compared to the other cytologies (p < 0.05).
CONCLUSION
Vascularity is not a useful parameter for distinguishing malignant from benign thyroid nodules. However, RI and PI values are useful in distinguishing malignant from benign thyroid nodules.
MeSH Terms
-
Adult
Aged
Biopsy, Fine-Needle
Chi-Square Distribution
Diagnosis, Differential
Female
Humans
Male
Middle Aged
Prospective Studies
ROC Curve
Sensitivity and Specificity
Statistics, Nonparametric
Thyroid Neoplasms/pathology/*ultrasonography
Thyroid Nodule/pathology/*ultrasonography
*Ultrasonography, Doppler, Duplex
Ultrasonography, Interventional
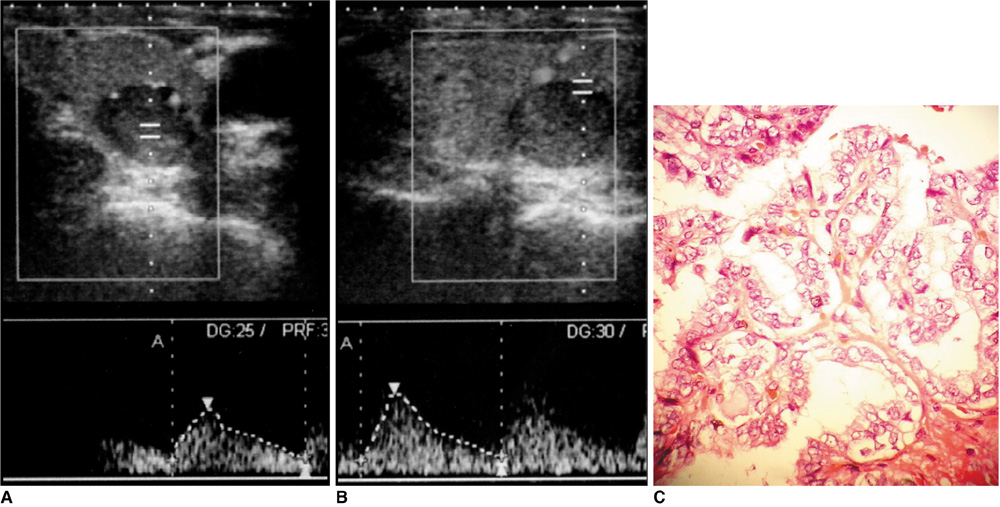
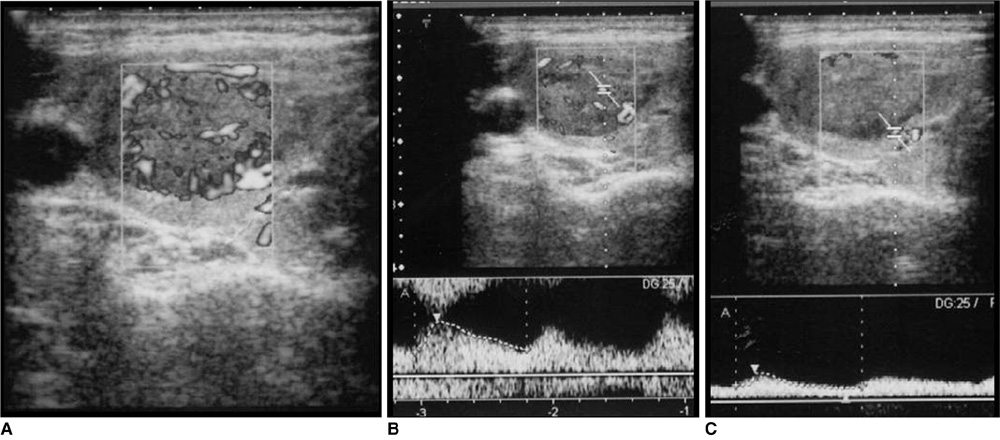
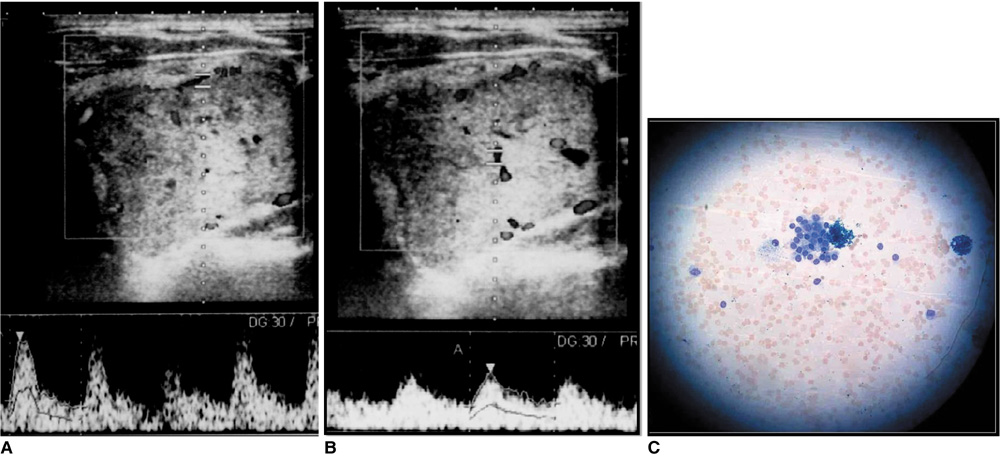
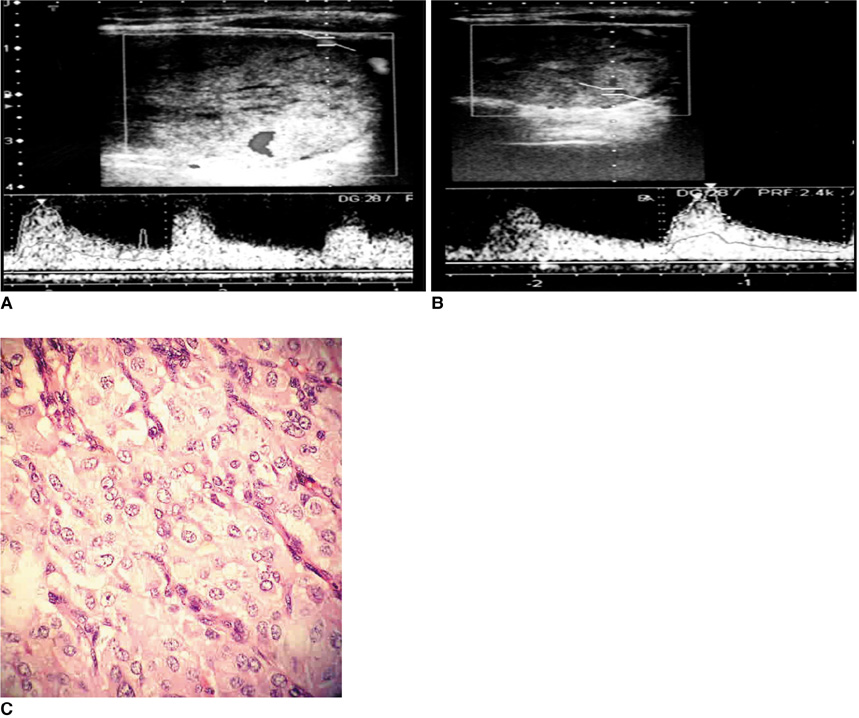
Figure
Cited by 1 articles
-
Complementary Role of Elastography Using Carotid Artery Pulsation in the Ultrasonographic Assessment of Thyroid Nodules: A Prospective Study
Soo Yeon Hahn, Jung Hee Shin, Eun Young Ko, Jung Min Bae, Ji Soo Choi, Ko Woon Park
Korean J Radiol. 2018;19(5):992-999. doi: 10.3348/kjr.2018.19.5.992.
Reference
-
1. Chammas MC, Gerhard R, de Oliveira IR, Widman A, de Barros N, Durazzo M, et al. Thyroid nodules: evaluation with power Doppler and duplex Doppler ultrasound. Otolaryngol Head Neck Surg. 2005. 132:874–882.2. Fukunari N, Nagahama M, Sugino K, Mimura T, Ito K, Ito K. Clinical evaluation of color Doppler imaging for the differential diagnosis of thyroid follicular lesions. World J Surg. 2004. 28:1261–1265.3. Chan BK, Desser TS, McDougall IR, Weigel RJ, Jeffrey RB Jr. Common and uncommon sonographic features of papillary thyroid carcinoma. J Ultrasound Med. 2003. 22:1083–1090.4. Iannuccilli JD, Cronan JJ, Monchik JM. Risk of malignancy of thyroid nodules as assessed by sonographic criteria: the need for biopsy. J Ultrasound Med. 2004. 23:1455–1464.5. Bakhshaee M, Davoudi Y, Mehrabi M, Layegh P, Mirsadaee S, Rad MP, et al. Vascular pattern and spectral parameters of power Doppler ultrasound as predictors of malignancy risk in thyroid nodules. Laryngoscope. 2008. 118:2182–2186.6. Decherd ME, Ryan MW. Evaluation of the thyroid nodule. Grand Rounds Presentation, UTMB, Dept. of otolaryngology. 2002.7. Papini E, Guglielmi R, Bianchini A, Crescenzi A, Taccogna S, Nardi F, et al. Risk of malignancy in nonpalpable thyroid nodules: predictive value of ultrasound and color-Doppler features. J Clin Endocrinol Metab. 2002. 87:1941–1946.8. Peccin S, de Castsro JA, Furlanetto TW, Furtado AP, Brasil BA, Czepielewski MA. Ultrasonography: is it useful in the diagnosis of cancer in thyroid nodules? J Endocrinol Invest. 2002. 25:39–43.9. Frates MC, Benson CB, Charboneau JW, Cibas ES, Clark OH, Coleman BG, et al. Management of thyroid nodules detected at US: Society of Radiologists in Ultrasound consensus conference statement. Radiology. 2005. 237:794–800.10. Appetecchia M, Solivetti FM. The association of colour flow Doppler sonography and conventional ultrasonography improves the diagnosis of thyroid carcinoma. Horm Res. 2006. 66:249–256.11. Wienke JR, Chong WK, Fielding JR, Zou KH, Mittelstaedt CA. Sonographic features of benign thyroid nodules: interobserver reliability and overlap with malignancy. J Ultrasound Med. 2003. 22:1027–1031.12. Stacul F, Bertolotto M, De Gobbis F, Calderan L, Cioffi V, Romano A, et al. US, colour-Doppler US and fine-needle aspiration biopsy in the diagnosis of thyroid nodules. Radiol Med. 2007. 112:751–762.13. Moon HJ, Kwak JY, Kim MJ, Son EJ, Kim EK. Can vascularity at power Doppler US help predict thyroid malignancy? Radiology. 2010. 255:260–269.14. Frates MC, Benson CB, Doubilet PM, Kunreuther E, Contreras M, Cibas ES, et al. Prevalence and distribution of carcinoma in patients with solitary and multiple thyroid nodules on sonography. J Clin Endocrinol Metab. 2006. 91:3411–3417.15. Gul K, Ersoy R, Dirikoc A, Korukluoglu B, Ersoy PE, Aydin R, et al. Ultrasonographic evaluation of thyroid nodules: comparison of ultrasonographic, cytological, and histopathological findings. Endocrine. 2009. 36:464–472.16. Tamsel S, Demirpolat G, Erdogan M, Nart D, Karadeniz M, Uluer H, et al. Power Doppler US patterns of vascularity and spectral Doppler US parameters in predicting malignancy in thyroid nodules. Clin Radiol. 2007. 62:245–251.17. Chammas MC, de Araujo Filho VJ, Moysés RA, Brescia MD, Mulatti GC, Brandão LG, et al. Predictive value for malignancy in the finding of microcalcifications on ultrasonography of thyroid nodules. Head Neck. 2008. 30:1206–1210.18. Moon WJ, Jung SL, Lee JH, Na DG, Baek JH, Lee YH, et al. Benign and malignant thyroid nodules: US differentiation--multicenter retrospective study. Radiology. 2008. 247:762–770.19. Gharib H, Papini E, Paschke R. Thyroid nodules: a review of current guidelines, practices, and prospects. Eur J Endocrinol. 2008. 159:493–505.20. Ron E, Lubin JH, Shore RE, Mabuchi K, Modan B, Pottern LM, et al. Thyroid cancer after exposure to external radiation: a pooled analysis of seven studies. Radiat Res. 1995. 141:259–277.21. Belfiore A, La Rosa GL, La Porta GA, Giuffrida D, Milazzo G, Lupo L, et al. Cancer risk in patients with cold thyroid nodules: relevance of iodine intake, sex, age and multinodularity. Am J Med. 1992. 93:363–369.22. Franklyn JA, Daykin J, Young J, Oates GD, Sheppard MC. Fine needle aspiration cytology in diffuse or multinodular goitre compared with solitary thyroid nodules. BMJ. 1993. 307:240.23. Schneider AB, Bekerman C, Leland J, Rosengarten J, Hyun H, Collins B, et al. Thyroid nodules in the follow-up of irradiated individuals: comparison of thyroid ultrasound with scanning and palpation. J Clin Endocrinol Metab. 1997. 82:4020–4027.24. Ezzat S, Sarti DA, Cain DR, Braunstein GD. Thyroid incidentalomas. Prevalence by palpation and ultrasonography. Arch Intern Med. 1994. 154:1838–1840.25. Tomimori E, Pedrinola F, Cavaliere H, Knobel M, Medeiros-Neto G. Prevalence of incidental thyroid disease in a relatively low iodine intake area. Thyroid. 1995. 5:273–276.26. Brander A, Viikinkoski P, Tuuhea J, Voutilainen L, Kivisaari L. Clinical versus ultrasound examination of the thyroid gland in common clinical practice. J Clin Ultrasound. 1992. 20:37–42.27. Cappelli C, Castellano M, Pirola I, Cumetti D, Agosti B, Gandossi E, et al. The predictive value of ultrasound findings in the management of thyroid nodules. QJM. 2007. 100:29–35.28. Jung SL, Jung CK, Kim SH, Kang BJ, Ahn KJ, Kim BS, et al. Histopathologic findings related to the indeterminate or inadequate results of fine-needle aspiration biopsy and correlation with ultrasonographic findings in papillary thyroid carcinomas. Korean J Radiol. 2010. 11:141–148.29. Cappelli C, Pirola I, Cumetti D, Micheletti L, Tironi A, Gandossi E, et al. Is the anteroposterior and transverse diameter ratio of nonpalpable thyroid nodules a sonographic criteria for recommending fine-needle aspiration cytology? Clin Endocrinol (Oxf). 2005. 63:689–693.30. Shimamoto K, Sakuma S, Ishigaki T, Makino N. Intratumoral blood flow: evaluation with color-Doppler echography. Radiology. 1987. 165:683–685.31. Argalia G, D'Ambrosio F, Lucarelli F, Mignosi U, Giuseppetti GM, Passarini G, et al. Echo Doppler in the characterization of thyroid nodular disease. Radiol Med. 1995. 89:651–657. [Italian].32. De Nicola H, Szejnfeld J, Logullo AF, Wolosker AM, Souza LR, Chiferi V Jr. Flow pattern and vascular resistive index as predictors of malignancy risk in thyroid follicular neoplasms. J Ultrasound Med. 2005. 24:897–904.33. Kwak JY, Kim EK, Kim HJ, Kim MJ, Son EJ, Moon HJ. How to combine ultrasound and cytological information in decision making about thyroid nodules. Eur Radiol. 2009. 19:1923–1931.34. Ivanac G, Brkljacic B, Ivanac K, Huzjan R, Skreb F, Cikara I. Vascularisation of benign and malignant thyroid nodules: CD US evaluation. Ultraschall Med. 2007. 28:502–506.35. Yang TF, Wang JD, Luo HJ, Wang XY, Li FH. Relationship between ultrasonographic velocimetric parameters and microvessel density in patients with papillary thyroid carcinoma and its clinical significance. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2007. 42:126–129. [Chinese].36. Algin O. Spectral power Doppler ultrasound parameters: are they really significant? Laryngoscope. 2009. 119:1452.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Evaluation of Solid Breast Masses with Power Doppler Ultrasound
- Resistive Index in Breast Tumors: Usefulness on Differentiation between Benign and Malignant Lesions
- Elastography of the Thyroid Glands
- Color Doppler Image of Thyroid Nodule: Differentiation Between Benign and Malignant Lesion
- Ultrasonographic Findings of Thyroid Nodules: Differentiation between Malignant and Benign Nodules