Korean J Radiol.
2004 Dec;5(4):250-257. 10.3348/kjr.2004.5.4.250.
Optimization of Wet Radiofrequency Ablation Using a Perfused-Cooled Electrode: A Comparative Study in Ex Vivo Bovine Livers
- Affiliations
-
- 1Department of Radiology, and Institute of Radiation Medicine, Seoul National University College of Medicine, Korea. HANJK@RADCOM.SNU.AC.KR
- 2Clinical Research Institute, Seoul National University Hospital, Korea.
- 3Department of Radiology, Chungnam National University College of Medicine, Korea.
- KMID: 1118832
- DOI: http://doi.org/10.3348/kjr.2004.5.4.250
Abstract
OBJECTIVE
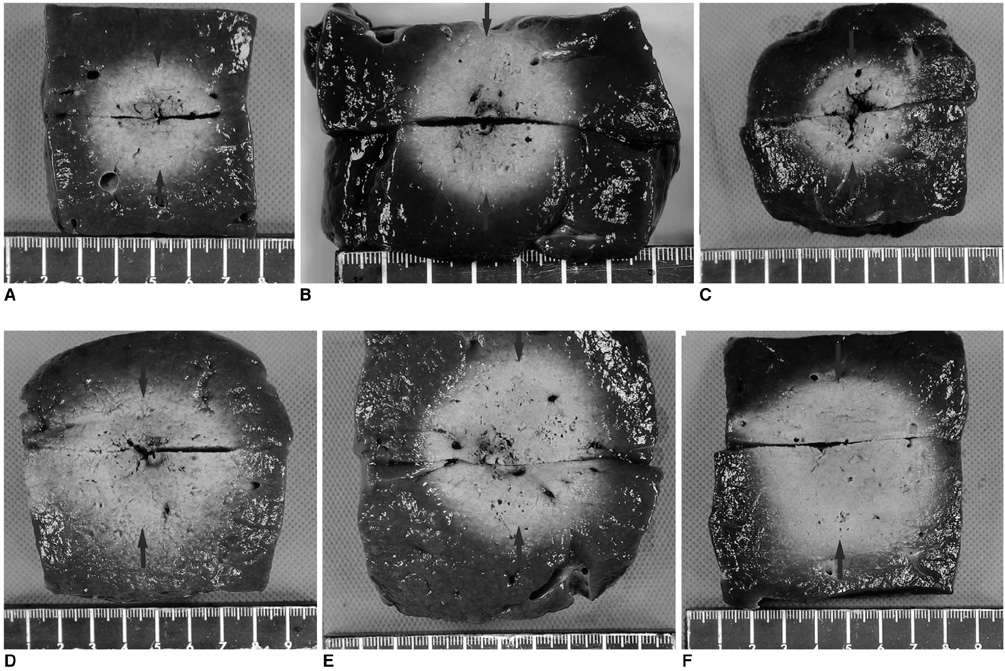
To determine the optimized protocol for wet monopolar radiofrequency ablation (RFA) using a perfused-cooled electrode to induce coagulation necrosis in the ex vivo bovine liver. MATER AND METHODS: Radiofrequency was applied to excised bovine livers in a monopolar mode using a 200W generator with an internally cooled electrode (groups A and B) or a perfused-cooled electrode (groups C, D, E, and F) at maximum power (150-200 W) for 10 minutes. A total of 60 ablation zones were created with six different regimens: group A - dry RFA using intra-electrode cooling; group B - dry RFA using intra-electrode cooling and a pulsing algorithm; group C - wet RFA using only interstitial hypertonic saline (HS) infusion; group D - wet RFA using interstitial HS infusion and a pulsing algorithm; group E - wet RFA using interstitial HS infusion and intra-electrode cooling; and group F - wet RFA using interstitial HS infusion, intra-electrode cooling and a pulsing algorithm. In groups C, D, E, and F, RFA was performed with the infusion of 6% HS through the perfused cooled electrode at a rate of 2 mL/minute. During RFA, we measured the tissue temperature at a distance of 15 mm from the electrode. The dimensions of the ablation zones and the changes in impedance, currents, and liver temperature during RFA were compared between these six groups. RESULTS: During RFA, the mean tissue impedances in groups A (243+/-88 omega) and C (252.5+/-108 omega) were significantly higher than those in groups B (85+/-18.7 omega), D (108.2+/-85 omega), E (70.0+/-16.3 omega), and F (66.5+/-7 omega) (p < 0.05). The mean currents in groups E and F were significantly higher than those in groups B and D, which were significantly higher than those in groups A and C (p < 0.05) : 520+/-425 mA in group A, 1163+/-34 mA in group B, 652.5+/-418 mA in group C, 842.5+/-773 mA in group D, 1665+/-295 mA in group E, and 1830+/-109 mA in group F. The mean volumes of the ablation regions in groups E and F were significantly larger than those in the other groups (p < 0.05) : 17.7+/-5.6 cm3 in group A, 34.5+/-3.0 cm3 in group B, 20.2+/-15.6 cm3 in group C, 36.1+/-19.5 cm3 in group D, 68.1+/-12.4 cm3 in group E, and 79.5+/-31 cm3 in group F. The final tissue temperatures at a distance of 15 mm from the electrode were higher in groups E and F than those in groups A, C, and D (p < 0.05) : 50+/-7.5 degreesC in group A, 66+/-13.6 degreesC in group B, 60+/-13.4 degreesC in group C, 61+/-12.7 degreesC in group D, 78+/-14.2 degreesC in group E, and 79+/-12.0 degreesC in group F. CONCLUSION: Wet monopolar RFA, using intra-electrode cooling and interstitial saline infusion, showed better performance in creating a large ablation zone than either dry RFA or wet RFA without intra-electrode cooling.
MeSH Terms
Figure
Reference
-
1. Lim HK. Radiofrequency thermal ablation of hepatocellular carcinomas. Korean J Radiol. 2000. 1:175–184.2. McGhana JP, Dodd GD 3rd. Radiofrequency ablation of the liver: current status. AJR Am J Roentgenol. 2001. 176:3–16.3. Curley SA, Izzo F, Delrio P, et al. Radiofrequency ablation of unresectable primary and metastatic hepatic malignancies: results in 123 patients. Ann Surg. 1999. 230:1–8.4. Lencioni RA, Allgaier HP, Cioni D, et al. Small hepatocellular carcinoma in cirrhosis: randomized comparison of radio-frequency thermal ablation versus percutaneous ethanol injection. Radiology. 2003. 228:235–240.5. Goldberg SN. Radiofrequency tumor ablation: principles and techniques. Eur J Ultrasound. 2001. 13:129–147.6. Goldberg SN, Solbiati L, Hahn PF, et al. Large-volume tissue ablation with radio frequency by using a clustered, internally cooled electrode technique: laboratory and clinical experience in liver metastases. Radiology. 1998. 209:371–379.7. Curley SA, Izzo F, Ellis LM, Vauthey JN, Vallone P. Radiofrequency ablation of hepatocellular cancer in 110 patients with cirrhosis. Ann Surg. 2000. 232:381–391.8. Denys AL, De Baere T, Kuoch V, et al. Radio-frequency tissue ablation of the liver: in vivo and ex vivo experiments with four different systems. Eur Radiol. 2003. 13:2346–2352.9. Dupuy DE, Goldberg SN. Image-guided radiofrequency tumor ablation: challenges and opportunities-part II. J Vasc Interv Radiol. 2001. 12:1135–1148.10. Gazelle GS, Goldberg SN, Solbiati L, Livraghi T. Tumor ablation with radio-frequency energy. Radiology. 2000. 217:633–646.11. Goldberg SN, Dupuy DE. Image guided radiofrequency tumor ablation: challenges and opportunities-part I. J Vasc Interv Radiol. 2001. 12:1021–1032.12. Dodd GD 3rd, Frank MS, Aribandi M, Chopra S, Chintapalli KN. Radiofrequency thermal ablation: computer analysis of the size of the thermal injury created by overlapping ablations. AJR Am J Roentgenol. 2001. 177:777–782.13. Choi D, Lim HK, Kim MJ, et al. Overlapping ablation using a coaxial radiofrequency electrode and multiple cannulae system: experimental study in ex-vivo bovine liver. Korean J Radiol. 2003. 4:117–123.14. Solbiati L, Livraghi T, Goldberg SN, et al. Percutaneous radiofrequency ablation of hepatic metastases from colorectal cancer: long-term results in 117 patients. Radiology. 2001. 221:159–166.15. Livraghi T, Goldberg SN, Lazzaroni S, et al. Hepatocellular carcinoma: radiofrequency ablation of medium and large lesions. Radiology. 2000. 214:761–768.16. Komorizono Y, Oketani M, Sako K, et al. Risk factors for local recurrence of small hepatocellular carcinoma tumors after a single session, single application of percutaneous radiofrequency ablation. Cancer. 2003. 97:1253–1262.17. Leveillee RJ, Hoey MF. Radiofrequency interstitial tissue ablation: wet electrode. J Endourol. 2003. 17:563–577.18. Goldberg SN, Ahmed M, Gazelle GS, et al. Radiofrequency thermal ablation with NaCl solution injection: effect of electrical conductivity on tissue heating, and coagulation-phantom and porcine liver study. Radiology. 2001. 219:157–165.19. Lee JM, Kim YK, Lee YH, Kim SW, Li CA, Kim CS. Percutaneous radiofrequency thermal ablation with hypertonic saline injection: in-vivo study in a rabbit liver model. Korean J Radiol. 2003. 4:27–34.20. Lobo SM, Afzal KS, Ahmed M, Kruskal JB, Lenkinski RE, Goldberg SM. Radiofrequency ablation: modeling the enhanced temperature response to adjuvant NaCl pretreatment. Radiology. 2004. 230:175–182.21. Schmidt D, Trubenbach J, Brieger J, et al. Automated saline-enhanced radiofrequency thermal ablation: initial results in ex vivo bovine livers. AJR Am J Roentgenol. 2003. 180:163–165.22. Hansler J, Frieser M, Schaber S, et al. Radiofrequency ablation of hepatocellular carcinoma with a saline solution perfusion device: a pilot study. J Vasc Interv Radiol. 2003. 14:575–580.23. Kettenbach J, Kostler W, Rucklinger E, et al. Percutaneous saline-enhanced radiofrequency ablation of unresectable hepatic tumors: initial experience in 26 patients. AJR Am J Roentgenol. 2003. 180:1537–1545.24. Solbiati L, Goldberg SN, Ierace T, et al. Hepatic metastases: percutaneous radiofrequency ablation with cooled-tip electrodes. Radiology. 1997. 205:367–373.25. Goldberg SN, Stein M, Gazelle GS, Sheiman RG, Kruskal JB, Clouse ME. Percutaneous radiofrequency tissue ablation: optimization of pulsed-RF technique to increase coagulation necrosis. J Vasc interv Radiol. 1999. 10:907–916.26. Ni Y, Miao Y, Mulier S, Yu J, Baert AL, Marchal G. A novel "cooled-wet" electrode for radiofrequency ablation. Eur Radiol. 2000. 10:852–854.27. Miao Y, Ni Y, Yu J, Marchal G. A comparative study on validation of a novel cooled-wet electrode for radiofrequency liver ablation. Invest Radiol. 2000. 35:438–444.28. Lee JM, Han JK, Kim SH, et al. Saline-enhanced hepatic radiofrequency ablation using a perfused-cooled electrode: comparison of dual probe bipolar mode with monopolar and single probe bipolar modes. Korean J Radiol. 2004. 5:121–127.29. Lee JD, Lee JM, Kim SW, Kim CS, Mun WS. MR imaging-histopathologic correlation of radiofrequency thermal ablation lesion in a rabbit liver model: observation during acute and chronic stages. Korean J Radiol. 2001. 2:151–158.30. Geeddes LA, Baker LE. The specific resistance of biological materials-a compedium of data for the biomedical engineer and physiologist. Med Biol Eng. 1967. 5:271–293.31. Brieger J, Pereira PL, Trubenbach J, et al. In vivo efficiency of four commercial monopolar radiofrequency ablation systems: a comparative experimental study in pig liver. Invest Radiol. 2003. 38:609–616.32. Boehm T, Malich A, Goldberg SN, et al. Radiofrequency tumor ablation: internally cooled electrode versus saline-enhanced technique in an aggressive rabbit tumor model. Radiology. 2002. 222:805–813.33. Goldberg SN, Ahmed M. Minimally invasive image-guided therapies for hepatocellular carcinoma. J Clin Gastroenterol. 2002. 35:S115–S129.34. Garcea G, Lloyd TD, Aylott C, Maddern G, Berry DP. The emergent role of focal liver ablation techniques in the treatment of primary and secondary liver tumours. Eur J Cancer. 2003. 39:2150–2164.35. Lau WY, Leung TW, Yu SC, Ho SK. Percutaneous local ablative therapy for hepatocellular carcinoma: a review and look into the future. Ann Surg. 2003. 237:171–179.36. Tranberg KG. Percutaneous ablation of liver tumors. Best Pract Res Clin Gastroenterol. 2004. 18:125–145.37. Patterson EJ, Scudamore CH, Owen DA, Nagy AG, Buczkowski AK. Radiofrequency ablation of porcine liver in vivo: effects of blood flow and treatment time on lesion size. Ann Surg. 1998. 227:559–565.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of Wet Radiofrequency Ablation with Dry Radiofrequency Ablation and Radiofrequency Ablation Using Hypertonic Saline Preinjection: Ex Vivo Bovine Liver
- Hepatic Radiofrequency Ablation Using Multiple Probes: Ex Vivo and In Vivo Comparative Studies of Monopolar versus Multipolar Modes
- Saline-Enhanced Hepatic Radiofrequency Ablation Using a Perfused-Cooled Electrode: Comparison of Dual Probe Bipolar Mode with Monopolar and Single Probe Bipolar Modes
- Bipolar Radiofrequency Ablation Using Dual Internally Cooled Wet Electrodes: Experimental Study in Ex Vivo Bovine Liver
- Radio-frequency Ablation in Patients with Malignant Hepatic Tumor and Experimental Model: Comparison of Expandable Needle and Water-Cooled Needle