Korean J Radiol.
2003 Jun;4(2):79-84. 10.3348/kjr.2003.4.2.79.
Fire-Related Post-Traumatic Stress Disorder: Brain 1H-MR Spetroscopic Findings
- Affiliations
-
- 1Department of Radiology, Inha University Hospital College of Medicine, Incheon, Korea. freesoul@inha.ac.kr
- 2Department of Psychiatry, Inha University Hospital College of Medicine, Incheon, Korea.
- 3Department of Radiology, Gachon Medical School, Incheon, Korea.
- 4National Institute of Neurologic Disorders and Stroke, NIH, Bethesda, U.S.A.
- KMID: 1118809
- DOI: http://doi.org/10.3348/kjr.2003.4.2.79
Abstract
OBJECTIVE
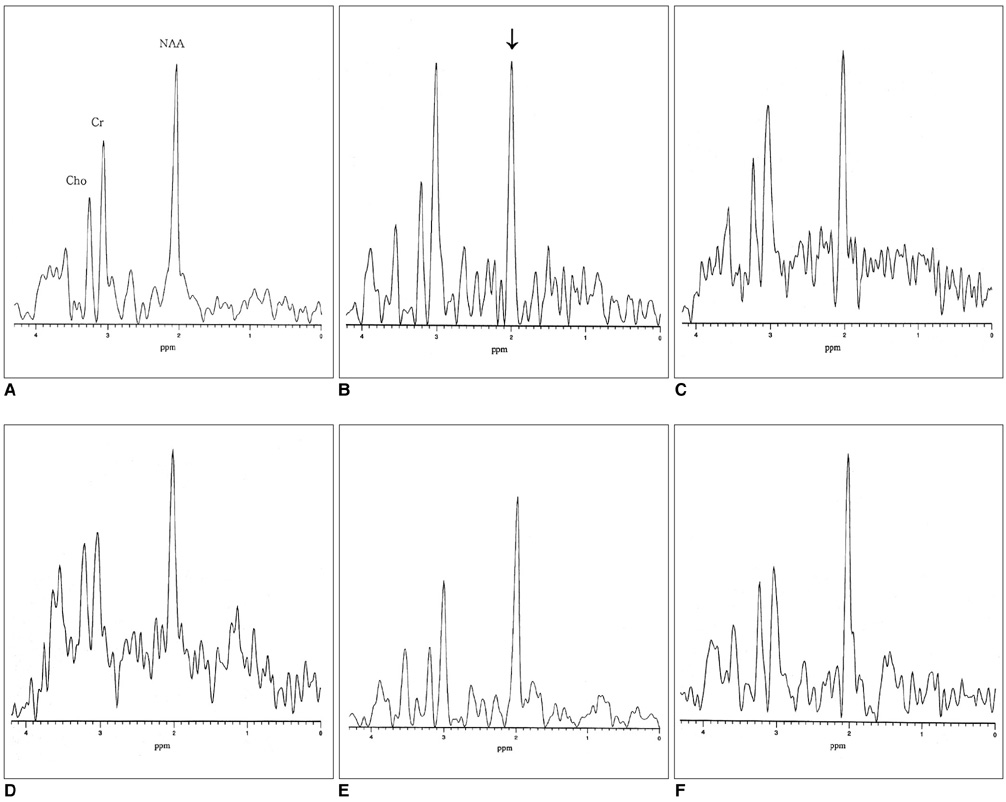
To investigate the MR imaging and 1H-MR spectroscopic findings of acute fire-related post-traumatic stress disorder (PTSD). MATERIALS AND METHODS: Sixteen patients (M: F=10: 6; mean age, 16 years) with fire-related PTSD underwent MR imaging and 1H-MR spectroscopy, and for control purposes, the procedures were repeated in eight age-matched normal volunteers. In all patients and controls, the regions of interest where data were acquired at MRS were the basal ganglia (BG), frontal periventricular white matter (FWM), and parietal periventricular white matter (PWM). RESULTS: In all patients with PTSD, MR images appeared normal. In contrast, MRS showed that in the BG, NAA/Cr ratios were significantly lower in patients than in volunteers. This decrease did not, however, show close correlation with the severity of the neuropsychiatric symptoms. In patients, neither NAA/Cr ratios in FWM nor PWM, nor Cho/Cr ratios in all three regions, were significantly different from those in the control group. CONCLUSION: Decreased NAA/Cr ratios in the BG, as seen at 1H-MRS, might be an early sign of acute fire-related PTSD.
Figure
Reference
-
1. Bremner D, Randall P, Scott TM, et al. MRI-based measurement of hippocampal volume in patients with combat-related post-traumatic stress disorder. Am J Psychiatry. 1995. 152:973–981.2. Gurvits TV, Shenton ME, Hokama H, et al. Magnetic resonance imaging study of hippocampal volume in chronic, combat-related posttraumatic stress disorder. Biol Psychiatry. 1996. 40:1091–1099.3. Bremner D, Randall P, Vermetten E, et al. Magnetic resonance imaging-based measurement of hippocampal volume in post-traumatic stress disorder related to childhood physical and sexual abuse - a preliminary report. Biol Psychiatry. 1997. 41:23–32.4. Rauch SL, van der Kolk BA, Fisler RE, et al. A symptom provocation study of posttraumatic stress disorder using positron emission tomography and script-driven imagery. Arch Gen Psychiatry. 1996. 53:380–387.5. Lee JH, Seo DW, Lee YS, et al. Proton magnetic resonance spectroscopy (1H-MRS) findings for the brain in patients with liver cirrhosis reflect the hepatic functional reserve. Am J Gastroenterol. 1999. 94:1–10.6. Ross BD, Jacobson S, Villamil F, et al. Subclinical hepatic encephalopathy: proton MR spectroscopic abnormalities. Radiology. 1994. 193:457–463.7. Lee CH, Lee JH, Kim JJ, et al. Cerebral metabolic abnormalities in congestive heart failure detected by proton magnetic resonance spectroscopy. J Am Coll Cardiol. 1999. 33:1196–1202.8. Chang KH, Kim HD, Park SW, et al. Usefulness of single-voxel proton MR spectroscopy in the evaluation of hippocampal sclerosis. Korean J Radiol. 2000. 1:25–32.9. Davidson J, Krishnan K, Charles H, et al. Magnetic resonance spectroscopy in social phobia: preliminary findings. J Clin Psychiatry. 1993. 54:Suppl. 19–25.10. Choi CG, Lee HK, Yoon JH. Localized proton MR spectroscopic evaluation of nonketotic hyperglycemia in an infant. Korean J Radiol. 2001. 2:239–242.11. Baik HM, Choe BY, Lee HK, Suh TS, Son BC, Lee JM. Metabolic alterations in Parkinson's disease after thalamotomy, as revealed by 1H-MR spectroscopy. Korean J Radiol. 2002. 3:180–188.12. Nasrallah HA, Skinner TE, Schmalbrock P, Pobitaille PM. Proton magnetic resonance spectroscopy of the hippocampal formation in schizophrenia: a pilot study. Br J Psychiatry. 1994. 165:481–485.13. Freeman TW, Caldwell D, Karson CN, Komoroski RA. In-vivo proton magnetic resonance spectroscopy of the medial temporal lobes of subjects with combat-related posttraumatic stress disorder. Magn Reson Med. 1998. 40:66–71.14. de Bellis MD, Keshavan MS, Spencer S, Hall J. N-acetyl aspartate concentration in the anterior cingulated of maltreated children and adolescents with PTSD. Am J Psychiatry. 2000. 157:1175–1177.15. Hahn OS, Ahn JH, Song SH, et al. Development of Korean version of structured clinical interview schedule for DSM-IV axis I disorder: inter-rater reliability. J Korean Neuropsychiatr Assoc. 2000. 39:362–372.16. Watson CG, Juba MP, Manifold V, Kucala T, Anderson PE. The PTSD interview: rationale, description, reliability and concurrent validity of a DSM-III-based technique. J Clin Psychol. 1991. 47:179–188.17. Kreis R, Ernst T, Ross BD. Development of the human brain: in-vivo quantification of metabolic and water content with proton magnetic resonance spectroscopy. Magn Reson Med. 1993. 30:424–437.18. Marshall I, Wardlaw J, Cannon J, Slattery J, Sellar RJ. Reproducibility of metabolite peak areas in 1H-MRS of brain. Magn Reson Imaging. 1996. 14:281–292.19. Strauss WL, Tsuruda JS, Richards TL. Partial volume effects in volume-localized phased-array proton spectroscopy of the temporal lobe. J Magn Reson Imaging. 1995. 4:433–436.20. Passe TJ, Charles HC, Rajagopalan P, Krishnan KR. Nuclear magnetic resonance spectroscopy: a review of neuropsychiatric applications. Prog Neuropsychopharmacol Biol Psychiatry. 1995. 19:541–563.21. Lim MK, Suh CH, Kim HJ, et al. Systemic lupus erythematosus: brain MR imaging and single-voxel hydrogen 1-MR spectroscopy. Radiology. 2000. 217:43–49.22. De Stefano N, Matthews PM, Arnold DL. Reversible decreases in N-acetylaspartate after acute brain injury. Magn Reson Med. 1995. 34:721–727.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Construction of a Post-traumatic Stress Model for Fire Fighters
- Effects of a Post-traumatic Stress Disorder Management Program on Firefighters' Post-traumatic Stress and Depression
- A Case Study of the Effects of Posttraumatic Stress Disorder on Operational Fire Service Personnel Within the Lancashire Fire and Rescue Service
- Post-Traumatic Stress Disorder, Depression and Anxiety among North Korean Refugees: A Meta-Analysis
- Posttraumatic Stress Disorder and Related Factors in Male Firefighters in a Metropolitan City