Thermal Ablation for Benign Thyroid Nodules: Radiofrequency and Laser
- Affiliations
-
- 1Department of Radiology and Research Institute of Radiology, University of Ulsan College of Medicine, Asan Medical Center, Seoul 138-736, Korea. radbaek@naver.com
- 2Endocrinology Division & Thyroid Disease Center, Arcispedale Santa Maria Nuova 42123, Reggio Emilia, Italy.
- 3Diagnostic Imaging and Interventional Radiology Department, Ospedale Regina Apostolorum 00041, Albano Laziale-Rome, Italy.
- 4Department of Radiology and Center for Imaging Science, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul 135-710, Korea.
- 5Department of Radiology, Human Medical Imaging and Intervention Center, Seoul 137-902, Korea.
- KMID: 1116437
- DOI: http://doi.org/10.3348/kjr.2011.12.5.525
Abstract
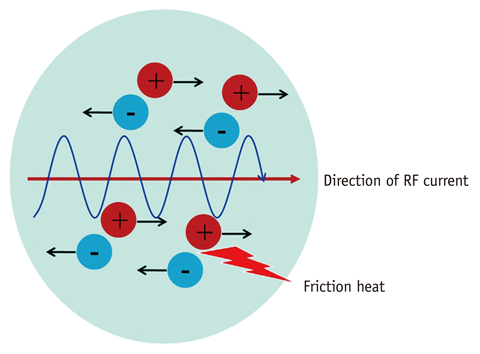
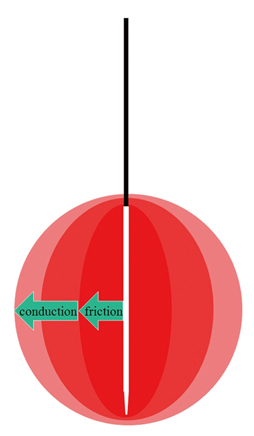
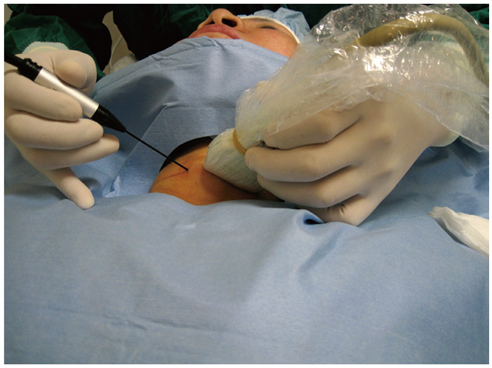
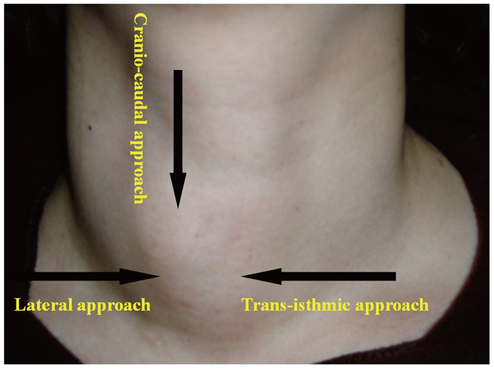
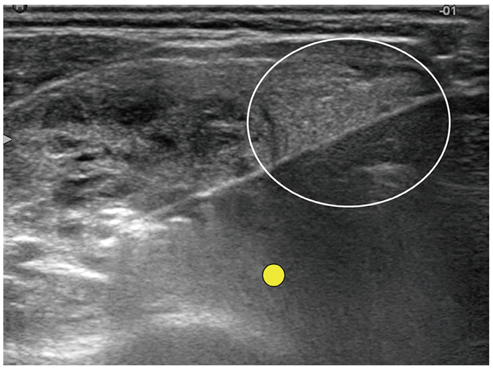
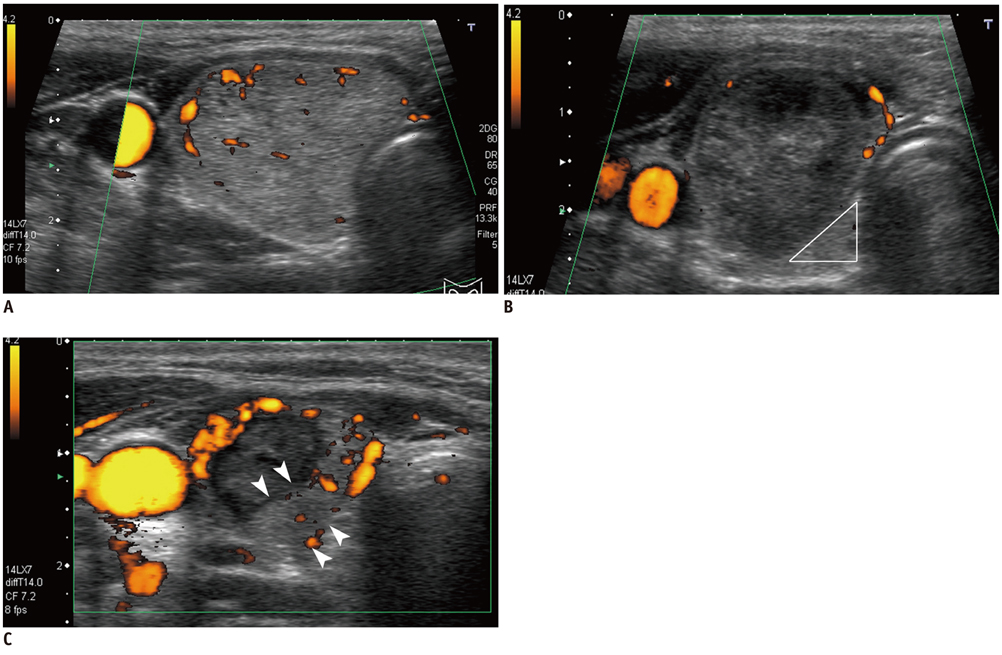
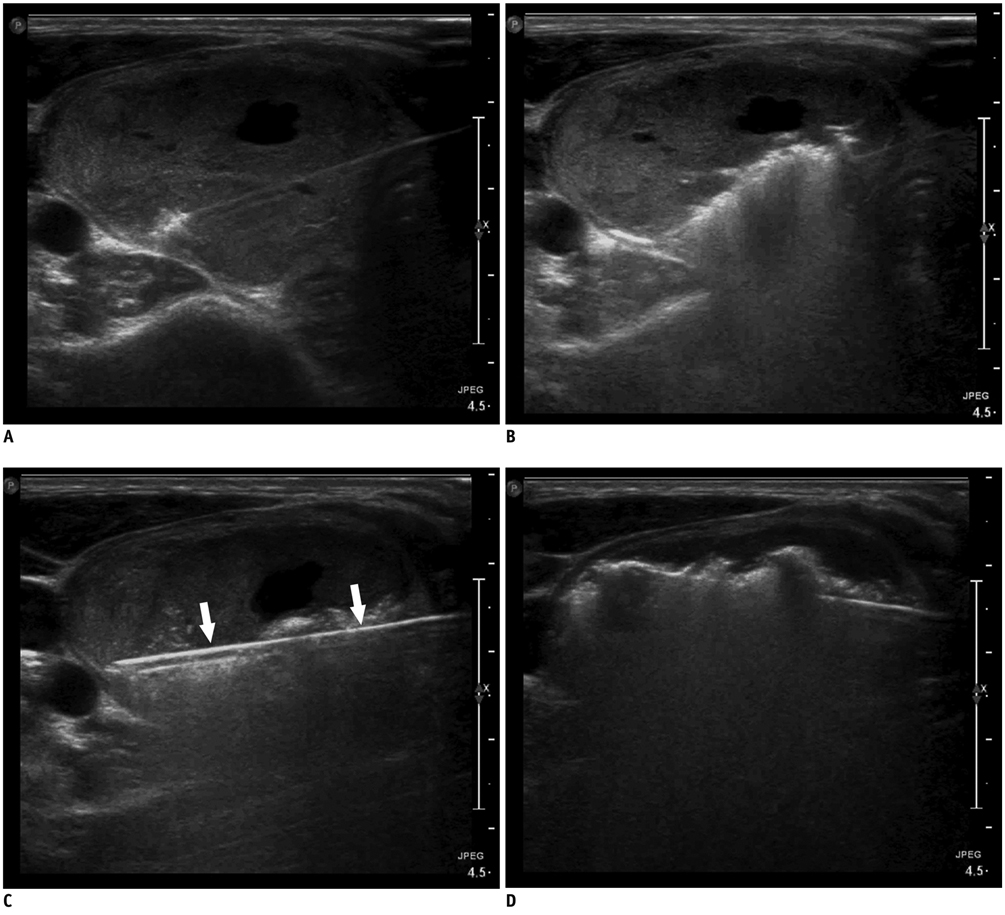
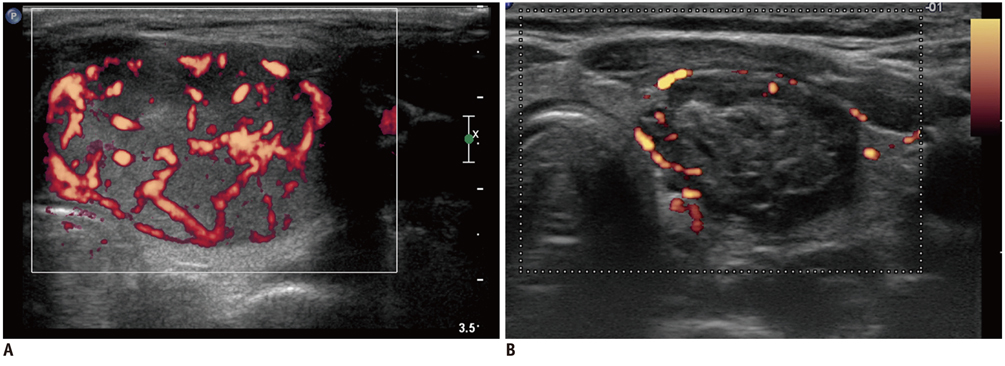
- Although ethanol ablation has been successfully used to treat cystic thyroid nodules, this procedure is less effective when the thyroid nodules are solid. Radiofrequency (RF) ablation, a newer procedure used to treat malignant liver tumors, has been valuable in the treatment of benign thyroid nodules regardless of the extent of the solid component. This article reviews the basic physics, techniques, applications, results, and complications of thyroid RF ablation, in comparison to laser ablation.
MeSH Terms
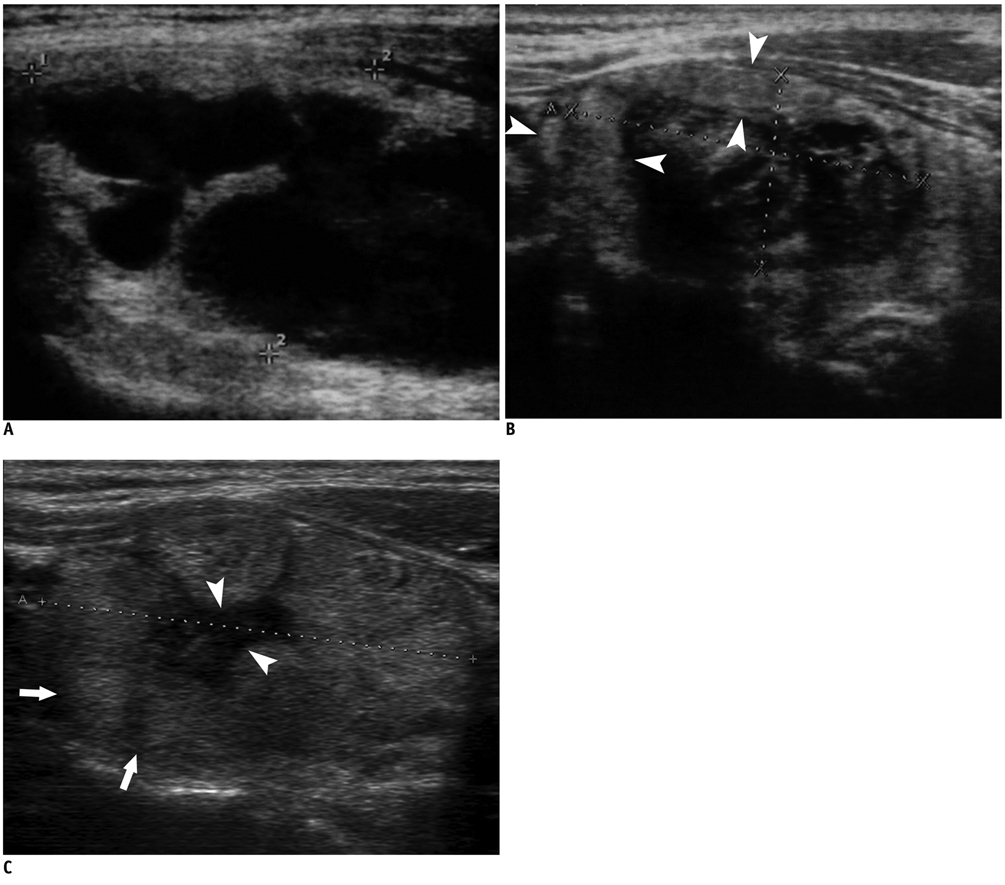
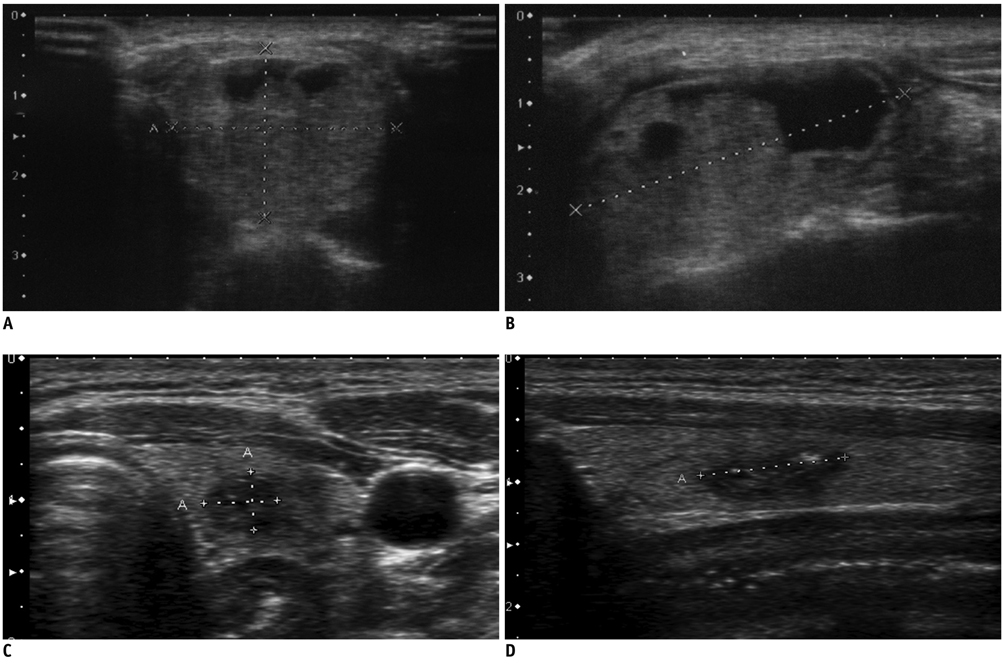
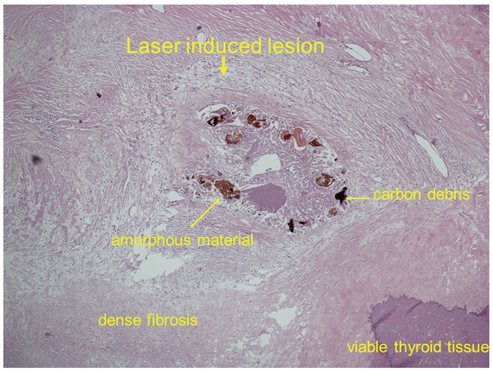
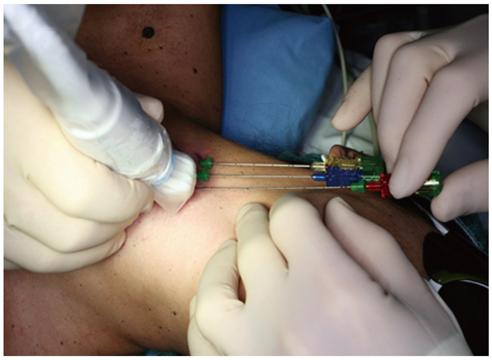
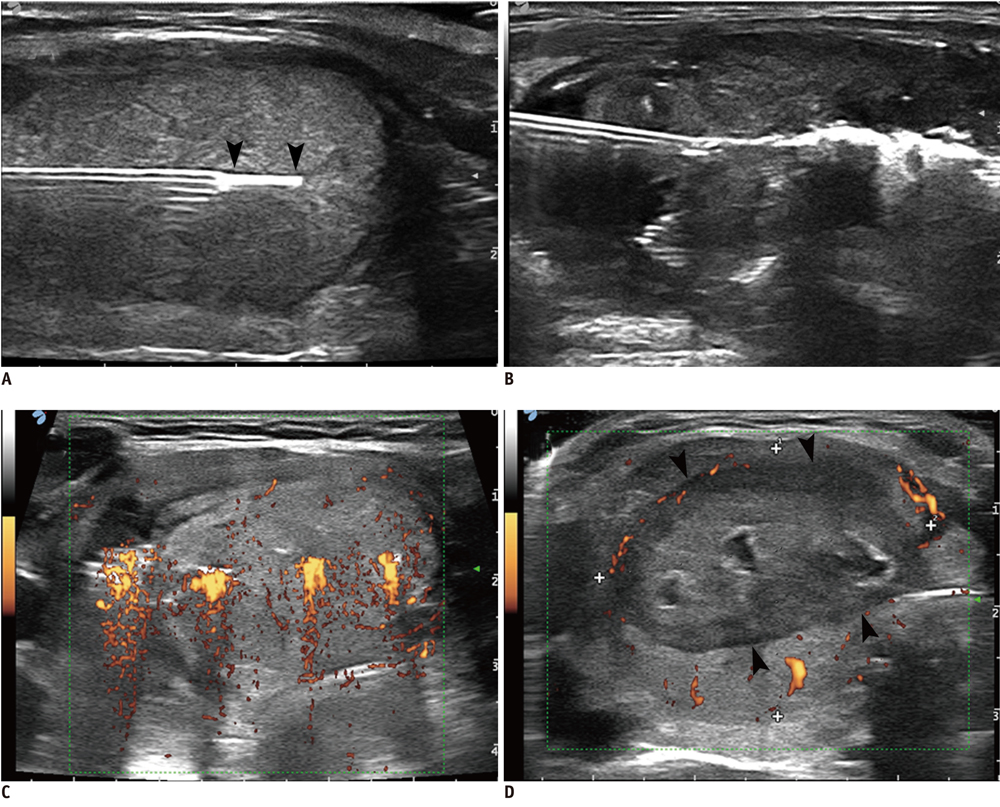
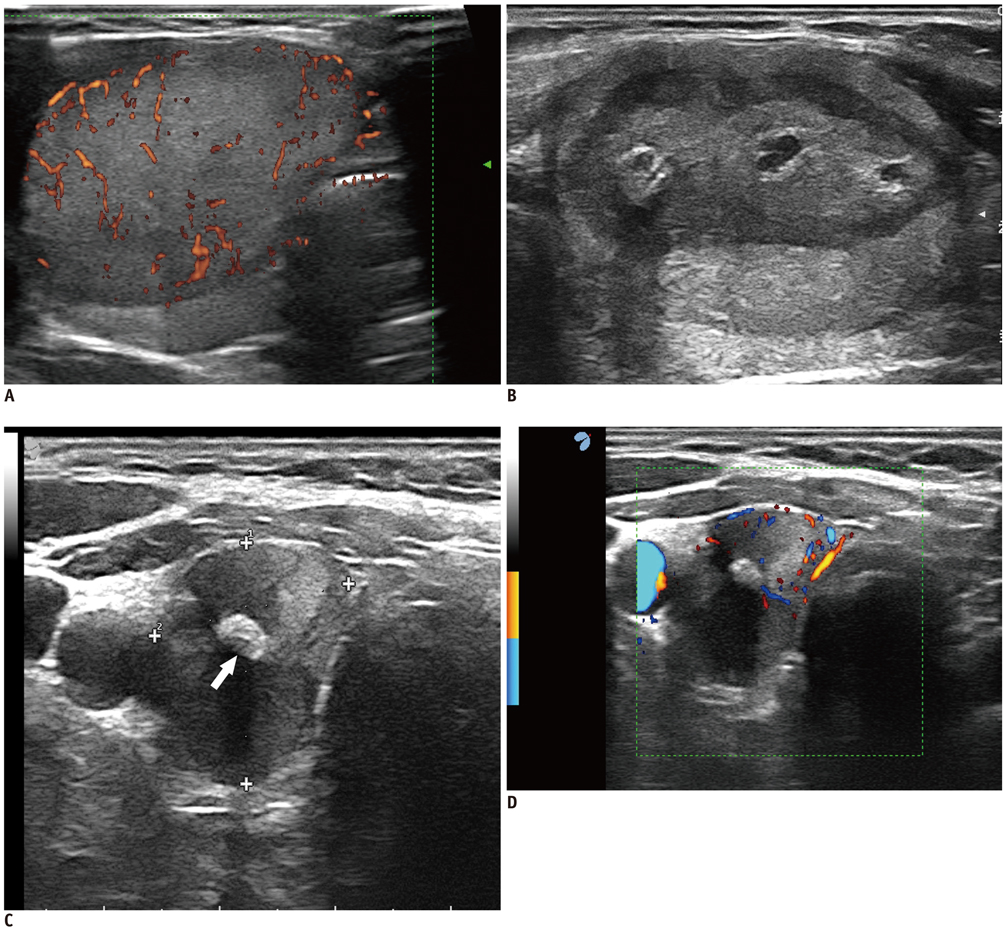
Figure
Cited by 10 articles
-
Long-Term Results of Thermal Ablation of Benign Thyroid Nodules: A Systematic Review and Meta-Analysis
Se Jin Cho, Jung Hwan Baek, Sae Rom Chung, Young Jun Choi, Jeong Hyun Lee
Endocrinol Metab. 2020;35(2):339-350. doi: 10.3803/EnM.2020.35.2.339.Predicting the Size of Benign Thyroid Nodules and Analysis of Associated Factors That Affect Nodule Size
Seok Ho Seo, Tae Hyun Kim, Soon Ho Kim, Seung Hyun Lee, Jong Taek Kim, Dae Won Park, Dong Chul Lee
Chonnam Med J. 2015;51(2):97-101. doi: 10.4068/cmj.2015.51.2.97.Long-Term Outcomes Following Thermal Ablation of Benign Thyroid Nodules as an Alternative to Surgery: The Importance of Controlling Regrowth
Jung Suk Sim, Jung Hwan Baek
Endocrinol Metab. 2019;34(2):117-123. doi: 10.3803/EnM.2019.34.2.117.Combination Therapy of Temporary Tracheal Stenting and Radiofrequency Ablation for Multinodular Thyroid Goiter with Airway Compression
Ji Hoon Shin, Jung Hwan Baek, Yeon-Mok Oh, Eun Ju Ha, Jeong Hyun Lee
Korean J Radiol. 2013;14(5):805-809. doi: 10.3348/kjr.2013.14.5.805.Moving-Shot versus Fixed Electrode Techniques for Radiofrequency Ablation: Comparison in an
Ex-Vivo Bovine Liver Tissue Model
Eun Ju Ha, Jung Hwan Baek, Jeong Hyun Lee
Korean J Radiol. 2014;15(6):836-843. doi: 10.3348/kjr.2014.15.6.836.Quality of Life in Patients Treated with Percutaneous Laser Ablation for Non-Functioning Benign Thyroid Nodules: A Prospective Single-Center Study
Silvia Oddo, Edineia Felix, Michele Mussap, Massimo Giusti
Korean J Radiol. 2018;19(1):175-184. doi: 10.3348/kjr.2018.19.1.175.Efficacy and Safety of Radiofrequency Ablation for Benign Thyroid Nodules: A Prospective Multicenter Study
So Lyung Jung, Jung Hwan Baek, Jeong Hyun Lee, Young Kee Shong, Jin Yong Sung, Kyu Sun Kim, Ducky Lee, Ji-hoon Kim, Seon Mi Baek, Jung Suk Sim, Dong Gyu Na
Korean J Radiol. 2018;19(1):167-174. doi: 10.3348/kjr.2018.19.1.167.2017 Thyroid Radiofrequency Ablation Guideline: Korean Society of Thyroid Radiology
Ji-hoon Kim, Jung Hwan Baek, Hyun Kyung Lim, Hye Shin Ahn, Seon Mi Baek, Yoon Jung Choi, Young Jun Choi, Sae Rom Chung, Eun Ju Ha, Soo Yeon Hahn, So Lyung Jung, Dae Sik Kim, Soo Jin Kim, Yeo Koon Kim, Chang Yoon Lee, Jeong Hyun Lee, Kwang Hwi Lee, Young Hen Lee, Jeong Seon Park, Hyesun Park, Jung Hee Shin, Chong Hyun Suh, Jin Yong Sung, Jung Suk Sim, Inyoung Youn, Miyoung Choi, Dong Gyu Na,
Korean J Radiol. 2018;19(4):632-655. doi: 10.3348/kjr.2018.19.4.632.Non-surgical, Image-guided Management of Benign Thyroid Nodules
Jung Hee Shin
J Korean Thyroid Assoc. 2014;7(2):111-117. doi: 10.11106/cet.2014.7.2.111.The Efficacy of Ultrasonography-Guided Radiofrequency Ablation in Patients With Benign Thyroid Goiters With a History of Unilateral Lobectomy
Hyo-Jun Kim, Ki-Nam Park, Seung-Won Lee
Clin Exp Otorhinolaryngol. 2020;13(3):312-314. doi: 10.21053/ceo.2020.00164.
Reference
-
1. Mazzaferri EL. Management of a solitary thyroid nodule. N Engl J Med. 1993. 328:553–559.2. Jeong WK, Baek JH, Rhim H, Kim YS, Kwak MS, Jeong HJ, et al. Radiofrequency ablation of benign thyroid nodules: safety and imaging follow-up in 236 patients. Eur Radiol. 2008. 18:1244–1250.3. Papini E, Guglielmi R, Bizzarri G, Pacella CM. Ultrasound-guided laser thermal ablation for treatment of benign thyroid nodules. Endocr Pract. 2004. 10:276–283.4. Shemen LJ, Strong EW. Complications after total thyroidectomy. Otolaryngol Head Neck Surg. 1989. 101:472–475.5. Papini E, Guglielmi R, Bizzarri G, Graziano F, Bianchini A, Brufani C, et al. Treatment of benign cold thyroid nodules: a randomized clinical trial of percutaneous laser ablation versus levothyroxine therapy or follow-up. Thyroid. 2007. 17:229–235.6. Dossing H, Bennedbaek FN, Karstrup S, Hegedus L. Benign solitary solid cold thyroid nodules: US-guided interstitial laser photocoagulation--initial experience. Radiology. 2002. 225:53–57.7. Pacella CM, Bizzarri G, Spiezia S, Bianchini A, Guglielmi R, Crescenzi A, et al. Thyroid tissue: US-guided percutaneous laser thermal ablation. Radiology. 2004. 232:272–280.8. Papini E, Pacella CM, Verde G. Percutaneous ethanol injection (PEI): what is its role in the treatment of benign thyroid nodules? Thyroid. 1995. 5:147–150.9. Spiezia S, Vitale G, Di Somma C, Pio Assanti A, Ciccarelli A, Lombardi G, et al. Ultrasound-guided laser thermal ablation in the treatment of autonomous hyperfunctioning thyroid nodules and compressive nontoxic nodular goiter. Thyroid. 2003. 13:941–947.10. Valcavi R, Frasoldati A. Ultrasound-guided percutaneous ethanol injection therapy in thyroid cystic nodules. Endocr Pract. 2004. 10:269–275.11. Sung JY, Baek JH, Kim YS, Jeong HJ, Kwak MS, Lee D, et al. One-step ethanol ablation of viscous cystic thyroid nodules. AJR Am J Roentgenol. 2008. 191:1730–1733.12. Yasuda K, Ozaki O, Sugino K, Yamashita T, Toshima K, Ito K, et al. Treatment of cystic lesions of the thyroid by ethanol instillation. World J Surg. 1992. 16:958–961.13. Zingrillo M, Torlontano M, Chiarella R, Ghiggi MR, Nirchio V, Bisceglia M, et al. Percutaneous ethanol injection may be a definitive treatment for symptomatic thyroid cystic nodules not treatable by surgery: five-year follow-up study. Thyroid. 1999. 9:763–767.14. Kim JH, Lee HK, Lee JH, Ahn IM, Choi CG. Efficacy of sonographically guided percutaneous ethanol injection for treatment of thyroid cysts versus solid thyroid nodules. AJR Am J Roentgenol. 2003. 180:1723–1726.15. Lee JH, Kim YS, Lee D, Choi H, Yoo H, Baek JH. Radiofrequency ablation (RFA) of benign thyroid nodules in patients with incompletely resolved clinical problems after ethanol ablation (EA). World J Surg. 2010. 34:1488–1493.16. Dupuy DE, Goldberg SN. Image-guided radiofrequency tumor ablation: challenges and opportunities--part II. J Vasc Interv Radiol. 2001. 12:1135–1148.17. Goldberg SN. Radiofrequency tumor ablation: principles and techniques. Eur J Ultrasound. 2001. 13:129–147.18. Kang TW, Rhim H, Kim EY, Kim YS, Choi D, Lee WJ, et al. Percutaneous radiofrequency ablation for the hepatocellular carcinoma abutting the diaphragm: assessment of safety and therapeutic efficacy. Korean J Radiol. 2009. 10:34–42.19. Lencioni R, Cioni D, Bartolozzi C. Percutaneous radiofrequency thermal ablation of liver malignancies: techniques, indications, imaging findings, and clinical results. Abdom Imaging. 2001. 26:345–360.20. Park SH, Yoon SK, Cho JH, Oh JY, Nam KJ, Kwon HJ, et al. Radiofrequency ablation treatment for renal cell carcinoma: early clinical experience. Korean J Radiol. 2008. 9:340–347.21. Rhim H, Goldberg SN, Dodd GD 3rd, Solbiati L, Lim HK, Tonolini M, et al. Essential techniques for successful radiofrequency thermal ablation of malignant hepatic tumors. Radiographics. 2001. 21 Spec No:S17–S35. discussion S36-19.22. Kanauchi H, Mimura Y, Kaminishi M. Percutaneous radiofrequency ablation of the thyroid guided by ultrasonography. Eur J Surg. 2001. 167:305–307.23. Baek JH, Jeong HJ, Kim YS, Kwak MS, Lee D. Radiofrequency Ablation for an Autonomously Functioning Thyroid Nodule. Thyroid. 2008. 18:675–676.24. Baek JH, Kim YS, Lee D, Huh JY, Lee JH. Benign predominantly solid thyroid nodules: prospective study of efficacy of sonographically guided radiofrequency ablation versus control condition. AJR Am J Roentgenol. 2010. 194:1137–1142.25. Baek JH, Moon WJ, Kim YS, Lee JH, Lee D. Radiofrequency ablation for the treatment of autonomously functioning thyroid nodules. World J Surg. 2009. 33:1971–1977.26. Deandrea M, Limone P, Basso E, Mormile A, Ragazzoni F, Gamarra E, et al. US-guided percutaneous radiofrequency thermal ablation for the treatment of solid benign hyperfunctioning or compressive thyroid nodules. Ultrasound Med Biol. 2008. 34:784–791.27. Spiezia S, Garberoglio R, Di Somma C, Deandrea M, Basso E, Limone PP, et al. Efficacy and safety of radiofrequency thermal ablation in the treatment of thyroid nodules with pressure symptoms in elderly patients. J Am Geriatr Soc. 2007. 55:1478–1479.28. Spiezia S, Garberoglio R, Milone F, Ramundo V, Caiazzo C, Assanti AP, et al. Thyroid nodules and related symptoms are stably controlled two years after radiofrequency thermal ablation. Thyroid. 2009. 19:219–225.29. Kim YS, Rhim H, Tae K, Park DW, Kim ST. Radiofrequency ablation of benign cold thyroid nodules: initial clinical experience. Thyroid. 2006. 16:361–367.30. Dupuy DE, Monchik JM, Decrea C, Pisharodi L. Radiofrequency ablation of regional recurrence from well-differentiated thyroid malignancy. Surgery. 2001. 130:971–977.31. Monchik JM, Donatini G, Iannuccilli J, Dupuy DE. Radiofrequency ablation and percutaneous ethanol injection treatment for recurrent local and distant well-differentiated thyroid carcinoma. Ann Surg. 2006. 244:296–304.32. Haemmerich D, Laeseke PF. Thermal tumour ablation: devices, clinical applications and future directions. Int J Hyperthermia. 2005. 21:755–760.33. Goldberg SN, Gazelle GS, Mueller PR. Thermal ablation therapy for focal malignancy: a unified approach to underlying principles, techniques, and diagnostic imaging guidance. AJR Am J Roentgenol. 2000. 174:323–331.34. Baek JH, Na DG, Lee JH, Jung SL, Sung JY, Sim J, et al. Korean Society of Thyroid Radiology recommendations for radiofrequency ablation of thyroid nodules. 2009. http://thyroidimaging.kr.35. Moon WJ, Baek JH, Jung SL, Kim DW, Kim EK, Kim JY, et al. Ultrasonography and the ultrasound-based management of thyroid nodules: consensus statement and recommendations. Korean J Radiol. 2011. 12:1–14.36. Moon WJ, Jung SL, Lee JH, Na DG, Baek J-H, Lee YH, et al. Benign and malignant thyroid nodules: US differentiation--multicenter retrospective study. Radiology. 2008. 247:762–770.37. Rhim H, Yoon KH, Lee JM, Cho Y, Cho JS, Kim SH, et al. Major complications after radio-frequency thermal ablation of hepatic tumors: spectrum of imaging findings. Radiographics. 2003. 23:123–134. discussion 134-126.38. Livraghi T, Solbiati L, Meloni MF, Gazelle GS, Halpern EF, Goldberg SN. Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology. 2003. 226:441–451.39. Baek JH, Kim YS, Sung JY, Choi H, Lee JH. Locoregional control of metastatic well differentiated thyroid cancer in the neck by ultrasonography-guided radiofrequency ablation. AJR Am J Roentgenol. 2011. 197:W331–W336.40. Dachman AH, McGehee JA, Beam TE, Burris JA, Powell DA. US-guided percutaneous laser ablation of liver tissue in a chronic pig model. Radiology. 1990. 176:129–133.41. Nikfarjam M, Muralidharan V, Malcontenti-Wilson C, Christophi C. Progressive microvascular injury in liver and colorectal liver metastases following laser induced focal hyperthermia therapy. Lasers Surg Med. 2005. 37:64–73.42. Nolsøe CP, Torp-Pedersen S, Burcharth F, Horn T, Pedersen S, Christensen NE, et al. Interstitial hyperthermia of colorectal liver metastases with a US-guided Nd-YAG laser with a diffuser tip: a pilot clinical study. Radiology. 1993. 187:333–337.43. Pacella CM, Rossi Z, Bizzarri G. Ultrasound-guided percutaneous laser ablation of liver tissue in a rabbit model. Eur Radiol. 1993. 3:26–32.44. Ritz JP, Lehmann KS, Zurbuchen U, Knappe V, Schumann T, Buhr HJ, et al. Ex vivo and in vivo evaluation of laser-induced thermotherapy for nodular thyroid disease. Lasers Surg Med. 2009. 41:479–486.45. Tranberg KG. Percutaneous ablation of liver tumours. Best Pract Res Clin Gastroenterol. 2004. 18:125–145.46. Pacella CM, Bizzarri G, Francica G, Bianchini A, De Nuntis S, Pacella S, et al. Percutaneous laser ablation in the treatment of hepatocellular carcinoma with small tumors: analysis of factors affecting the achievement of tumor necrosis. J Vasc Interv Radiol. 2005. 16:1447–1457.47. Gharib H, Papini E, Paschke R, Duick DS, Valcavi R, Hegedus L, et al. American Association of Clinical Endocrinologists, Associazione Medici Endocrinologi, and EuropeanThyroid Association Medical Guidelines for Clinical Practice for the Diagnosis and Management of Thyroid Nodules. Endocr Pract. 2010. 16:Suppl 1. 1–43.48. Pacella CM, Bizzarri G, Guglielmi R, Anelli V, Bianchini A, Crescenzi A, et al. Thyroid tissue: US-guided percutaneous interstitial laser ablation-a feasibility study. Radiology. 2000. 217:673–677.49. Dossing H, Bennedbaek FN, Hegedus L. Beneficial effect of combined aspiration and interstitial laser therapy in patients with benign cystic thyroid nodules: a pilot study. Br J Radiol. 2006. 79:943–947.50. Cakir B, Topaloglu O, Gul K, Agac T, Aydin C, Dirikoc A, et al. Effects of percutaneous laser ablation treatment in benign solitary thyroid nodules on nodule volume, thyroglobulin and anti-thyroglobulin levels, and cytopathology of nodule in 1 yr follow-up. J Endocrinol Invest. 2006. 29:876–884.51. Dossing H, Bennedbaek FN, Hegedus L. Ultrasound-guided interstitial laser photocoagulation of an autonomous thyroid nodule: the introduction of a novel alternative. Thyroid. 2003. 13:885–888.52. Dossing H, Bennedbaek FN, Hegedus L. Effect of ultrasound-guided interstitial laser photocoagulation on benign solitary solid cold thyroid nodules - a randomised study. Eur J Endocrinol. 2005. 152:341–345.53. Gambelunghe G, Fatone C, Ranchelli A, Fanelli C, Lucidi P, Cavaliere A, et al. A randomized controlled trial to evaluate the efficacy of ultrasound-guided laser photocoagulation for treatment of benign thyroid nodules. J Endocrinol Invest. 2006. 29:RC23–RC26.54. Valcavi R, Bertani A, Pesenti ML, Al Jandali Rifa' L, Frasoldati A, Formisano D, et al. Baskin HJ, Duick DS, Levine RA, editors. Laser and radiofrequency ablation procedures. Thyroid ultrasound and ultrasound-guided FNA biopsy. 2008. New York: Springer;191–218.55. Valcavi R, Riganti F, Bertani A, Formisano D, Pacella CM. Percutaneous laser ablation of cold benign thyroid nodules: a 3-year follow-up study in 122 patients. Thyroid. 2010. 20:1253–1261.56. Papini E, Bizzarri G, Pacella CM. Percutaneous laser ablation of benign and malignant thyroid nodules. Curr Opin Endocrinol Diabetes Obes. 2008. 15:434–439.57. Hegedus L. Therapy: a new nonsurgical therapy option for benign thyroid nodules? Nat Rev Endocrinol. 2009. 5:476–478.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Ultrasound (US)-Guided Ablation of Thyroid Nodules
- Long-Term Outcomes Following Thermal Ablation of Benign Thyroid Nodules as an Alternative to Surgery: The Importance of Controlling Regrowth
- Non-surgical, Image-guided Management of Benign Thyroid Nodules
- Effective and Safe Application of Radiofrequency Ablation for Benign Thyroid Nodules
- Non-surgical Management of Thyroid Nodules