Yonsei Med J.
2011 Sep;52(5):809-817. 10.3349/ymj.2011.52.5.809.
Effects of Multiple Drilling on the Ischemic Capital Femoral Epiphysis of Immature Piglets
- Affiliations
-
- 1Department of Orthopedic Surgery, Yonsei University College of Medicine, Seoul, Korea. leeks@yuhs.ac
- KMID: 1108075
- DOI: http://doi.org/10.3349/ymj.2011.52.5.809
Abstract
- PURPOSE
This study investigated the effects of multiple drilling on the immature capital femoral epiphysis following ischemic injury in a piglet model.
MATERIALS AND METHODS
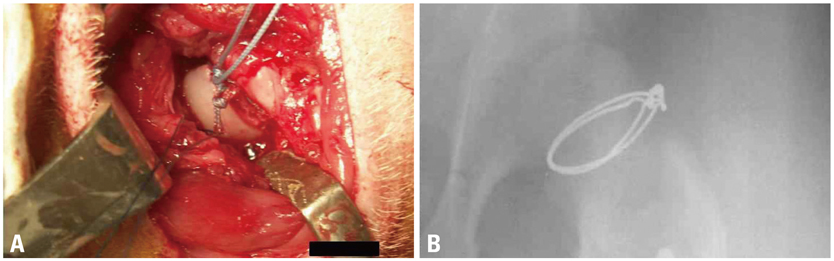
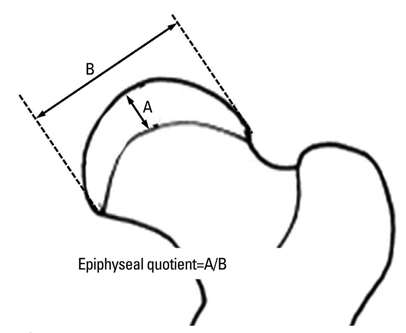
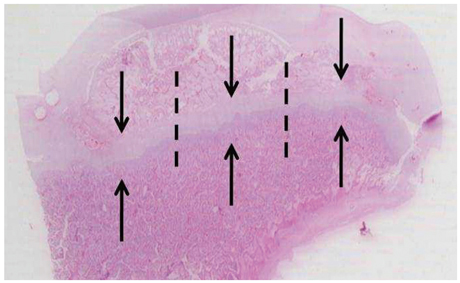
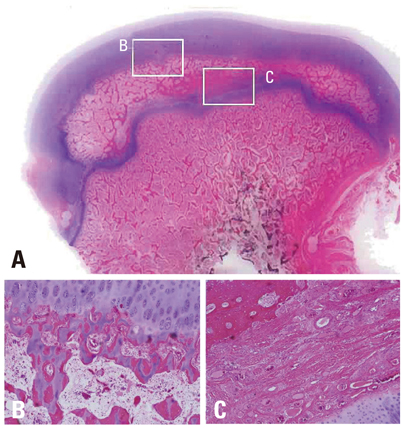
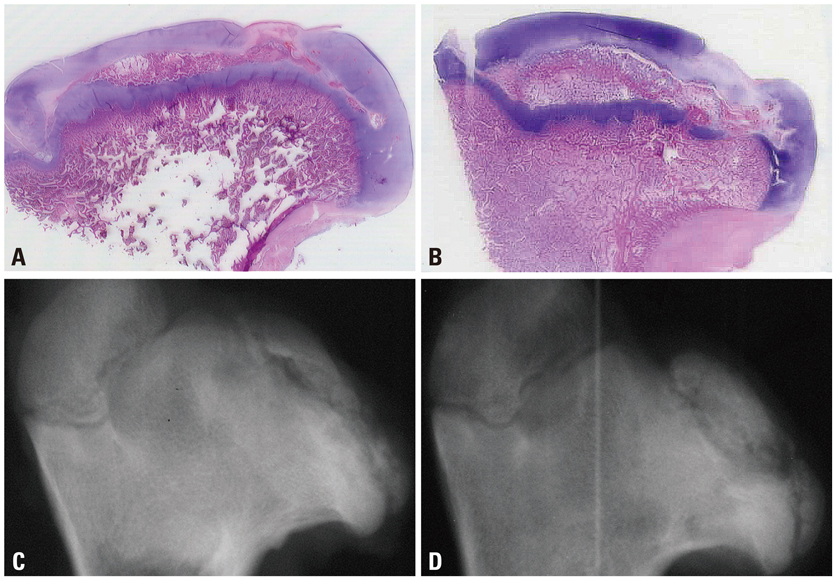
Ischemic necrosis of capital femoral epiphysis was induced bilaterally in 12 piglets using a cervical ligation method. Three weeks later, medial, central, and lateral 3 drill holes were made on the left femoral head using 0.062" K-wire. At 3, 6, 9, and 12 weeks following the multiple drilling, femoral heads were harvested from each three piglets. On histologic examination, percent of revascularization, percent of osteoblast surface, capital femoral epiphyseal quotient and proximal femoral growth plate height were evaluated. Untreated right femoral heads served as control.
RESULTS
While percent of revascularization of left capital femoral epiphysis with multiple drilling was significantly higher than untreated control side (p<0.001), percent of osteoblast surface, capital femoral epiphyseal quotient and proximal femoral growth plate height showed no significant difference.
CONCLUSION
This study indicates that multiple drilling could promote revascularization of ischemic capital femoral epiphysis, and multiple drilling does not appear to produce bony physeal bars at short-term, if using small diameter drill. However, multiple drilling alone does not seem to prevent femoral head deformity or to promote new bone formation.
MeSH Terms
Figure
Cited by 2 articles
-
Altered Cellular Kinetics in Growth Plate according to Alterations in Weight Bearing
Hoon Park, Sun Young Kong, Hyun Woo Kim, Ick Hwan Yang
Yonsei Med J. 2012;53(3):618-624. doi: 10.3349/ymj.2012.53.3.618.Altered Cellular Kinetics in the Growth Plate of the Femoral Head of Spontaneously Hypertensive Rats
Hoon Park, Sun Young Kong, Hyun Woo Kim
Yonsei Med J. 2012;53(3):625-633. doi: 10.3349/ymj.2012.53.3.625.
Reference
-
1. Rowe SM, Jung ST, Lee KB, Bae BH, Cheon SY, Kang KD. The incidence of Perthes' disease in Korea: a focus on differences among races. J Bone Joint Surg Br. 2005. 87:1666–1668.2. Thompson GH, Salter RB. Legg-Calvé-Perthes disease. Current concepts and controversies. Orthop Clin North Am. 1987. 18:617–613.3. Waldenstrom H. The first stages of coxa plana. J Bone Joint Surg Am. 1938. 20:559–566.
Article4. Lloyd-Roberts GC, Catterall A, Salamon PB. A controlled study of the indications for and the results of femoral osteotomy in Perthes' disease. J Bone Joint Surg Br. 1976. 58:31–36.
Article5. Herring JA, Kim HT, Browne R. Legg-Calve-Perthes disease. Part II: Prospective multicenter study of the effect of treatment on outcome. J Bone Joint Surg Am. 2004. 86-A:2121–2134.6. Willett K, Hudson I, Catterall A. Lateral shelf acetabuloplasty: an operation for older children with Perthes' disease. J Pediatr Orthop. 1992. 12:563–568.7. Joseph B, Mulpuri K, Varghese G. Perthes' disease in the adolescent. J Bone Joint Surg Br. 2001. 83:715–720.
Article8. Song WS, Yoo JJ, Kim YM, Kim HJ. Results of multiple drilling compared with those of conventional methods of core decompression. Clin Orthop Relat Res. 2007. 454:139–146.
Article9. Pohl J. [A contribution to covered drilling in perthes' disease]. Arch Orthop Unfallchir. 1964. 56:661–663.10. Baksi DP. Palliative operations for painful old Perthes' disease. Int Orthop. 1995. 19:46–50.
Article11. Bozsan EJ. A new treatment of intracapsular fractures of the neck of the femur and Calve-Legg-Perthes' disease. J Bone Joint Surg Am. 1932. 14:884–887.12. Kim HK, Su PH, Qiu YS. Histopathologic changes in growth-plate cartilage following ischemic necrosis of the capital femoral epiphysis. An experimental investigation in immature pigs. J Bone Joint Surg Am. 2001. 83-A:688–697.13. Kim HK, Morgan-Bagley S, Kostenuik P. RANKL inhibition: a novel strategy to decrease femoral head deformity after ischemic osteonecrosis. J Bone Miner Res. 2006. 21:1946–1954.
Article14. Kim HK, Randall TS, Bian H, Jenkins J, Garces A, Bauss F. Ibandronate for prevention of femoral head deformity after ischemic necrosis of the capital femoral epiphysis in immature pigs. J Bone Joint Surg Am. 2005. 87:550–557.
Article15. Heyman CH, Herndon CH. Legg-Perthes disease; a method for the measurement of the roentgenographic result. J Bone Joint Surg Am. 1950. 32:767–778.16. Kienzle L. [Therapy of coxa vara epiphysaria and Perthes' disease by the Beck's drilling-operation]. Z Orthop Ihre Grenzgeb. 1953. 83:270–275.17. Chung SM. The arterial supply of the developing proximal end of the human femur. J Bone Joint Surg Am. 1976. 58:961–970.
Article18. Atsumi T, Kuroki Y, Yamano K. A microangiographic study of idiopathic osteonecrosis of the femoral head. Clin Orthop Relat Res. 1989. 186–194.
Article19. Dahners LE, Hillsgrove DC. The effects of drilling on revascularization and new bone formation in canine femoral heads with avascular necrosis: an initial study. J Orthop Trauma. 1989. 3:309–312.
Article20. Janarv PM, Wikström B, Hirsch G. The influence of transphyseal drilling and tendon grafting on bone growth: an experimental study in the rabbit. J Pediatr Orthop. 1998. 18:149–154.
Article21. Kenzora JE, Steele RE, Yosipovitch ZH, Glimcher MJ. Experimental osteonecrosis of the femoral head in adult rabbits. Clin Orthop Relat Res. 1978. 8–46.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Erratum to "Effects of Multiple Drilling on the Ischemic Capital Femoral Epiphysis of Immature Piglets" by Gong SY, et al. (Yonsei Med J 2011;52:809-17.)
- Effect of Curettage and DBM-CaSO4 Graft for the Treatment of Ischemic Necrosis of the Capital Femoral Epiphysis in Immature Pigs
- Pathologic Separation of Capital Femoral Epiphysis due to an Osteosarcoma
- A Clinical Study of Slipped Capital Femoral epiphysis
- Responsible Factors for Femoral Shortening in Piglet Legg-Calve-Perthes Disease Models