Yonsei Med J.
2011 Sep;52(5):739-745. 10.3349/ymj.2011.52.5.739.
The Positive Association between Peripheral Blood Cell Counts and Bone Mineral Density in Postmenopausal Women
- Affiliations
-
- 1Department of Internal Medicine, Armed Forces Seoul Hospital, Seoul, Korea. khl2876@gmail.com
- 2Department of Internal Medicine, Seoul National University Hospital, Seoul, Korea.
- 3Department of Internal Medicine, Hanyang University Guri Hospital, Guri, Korea.
- 4Department of Preventive Medicine, The Armed Forces Medical Commands, Seongnam, Korea.
- KMID: 1108065
- DOI: http://doi.org/10.3349/ymj.2011.52.5.739
Abstract
- PURPOSE
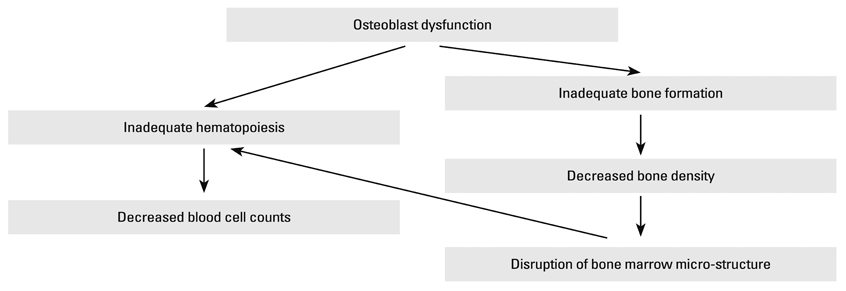
Accumulating evidence has shown a close connection between hematopoiesis and bone formation. Our aim was to evaluate the association between peripheral blood cell counts and bone mineral density (BMD) in a sample of postmenopausal women.
MATERIALS AND METHODS
three hundreds thirty eight healthy postmenopausal women who underwent BMD measurement during their health check-up were investigated. BMD was measured by dual energy X-ray asorptiometry at L1-L4 spine, femoral neck and total proximal femur. BMD was expressed as a T-score: among T-scores obtained from three different sites (L1-L4 spine, femoral neck and total proximal femur), the lowest T-score was considered to be the subject's T-score.
RESULTS
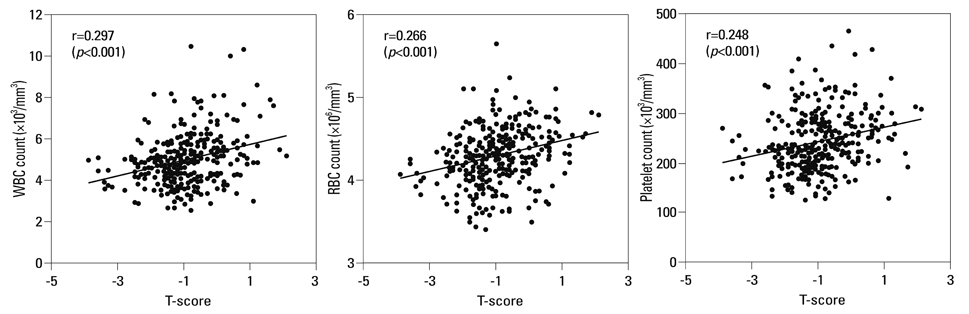
The prevalence of osteopenia and osteoporosis diagnosed by T-score in the study participants were 49.4% (167/338) and 5.0% (17/338), respectively. Peripheral blood white blood cell (WBC), red blood cell (RBC) and platelet counts had significant positive correlations with T-scores (p<0.001) upon simple linear regression analysis. A multiple linear regression analysis, after controlling of confounders including age, body weight, systolic blood pressure, alkaline phosphatase and creatinine, showed that WBC (beta=0.127; standard error=0.043; p=0.014), RBC (beta=0.192; standard error=0.139; p<0.001) and platelet (beta=0.097; standard error=0.001; p=0.050) counts still had significant positive association with T-scores.
CONCLUSION
The study results showed a positive relationship between blood cell counts and bone mineral density in postmenopausal women, supporting the idea of a close connection between hematopoiesis and bone formation. The study results also suggest that blood cell counts could be a putative marker for estimating BMD in postmenopausal women.
Keyword
MeSH Terms
Figure
Reference
-
1. Raisz LG. Pathogenesis of osteoporosis: concepts, conflicts, and prospects. J Clin Invest. 2005. 115:3318–3325.
Article2. Manolagas SC, Jilka RL. Bone marrow, cytokines, and bone remodeling. Emerging insights into the pathophysiology of osteoporosis. N Engl J Med. 1995. 332:305–311.
Article3. Adams GB, Scadden DT. The hematopoietic stem cell in its place. Nat Immunol. 2006. 7:333–337.
Article4. Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003. 425:841–846.
Article5. Taichman RS, Emerson SG. Human osteoblasts support hematopoiesis through the production of granulocyte colony-stimulating factor. J Exp Med. 1994. 179:1677–1682.
Article6. Taichman RS, Reilly MJ, Emerson SG. Human osteoblasts support human hematopoietic progenitor cells in vitro bone marrow cultures. Blood. 1996. 87:518–524.
Article7. Visnjic D, Kalajzic I, Gronowicz G, Aguila HL, Clark SH, Lichtler AC, et al. Conditional ablation of the osteoblast lineage in Col2.3deltatk transgenic mice. J Bone Miner Res. 2001. 16:2222–2231.
Article8. Visnjic D, Kalajzic Z, Rowe DW, Katavic V, Lorenzo J, Aguila HL. Hematopoiesis is severely altered in mice with an induced osteoblast deficiency. Blood. 2004. 103:3258–3264.
Article9. Wu JY, Scadden DT, Kronenberg HM. Role of the osteoblast lineage in the bone marrow hematopoietic niches. J Bone Miner Res. 2009. 24:759–764.
Article10. WHO scientific group on the prevention and management of osteoporosis. WHO technical report series, 921. Prevention and management of osteoporosis: report of a WHO scientific group. 2000. Geneva: WHO Library.11. Taaffe DR, Cauley JA, Danielson M, Nevitt MC, Lang TF, Bauer DC, et al. Race and sex effects on the association between muscle strength, soft tissue, and bone mineral density in healthy elders: the Health, Aging, and Body Composition Study. J Bone Miner Res. 2001. 16:1343–1352.
Article12. Aguila HL, Rowe DW. Skeletal development, bone remodeling, and hematopoiesis. Immunol Rev. 2005. 208:7–18.
Article13. Taichman RS. Blood and bone: two tissues whose fates are intertwined to create the hematopoietic stem-cell niche. Blood. 2005. 105:2631–2639.
Article14. Hughes DE, Salter DM, Dedhar S, Simpson R. Integrin expression in human bone. J Bone Miner Res. 1993. 8:527–533.
Article15. Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003. 425:836–841.
Article16. Taichman RS, Emerson SG. The role of osteoblasts in the hematopoietic microenvironment. Stem Cells. 1998. 16:7–15.
Article17. Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, et al. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997. 89:755–764.
Article18. Chappard D, Legrand E, Audran M, Basle MF. [Histomorphometric measurement of the architecture of the trabecular bone in osteoporosis: comparative study of several methods]. Morphologie. 1999. 83:17–20.19. Shih TT, Chang CJ, Hsu CY, Wei SY, Su KC, Chung HW. Correlation of bone marrow lipid water content with bone mineral density on the lumbar spine. Spine (Phila Pa 1976). 2004. 29:2844–2850.
Article20. Rodríguez JP, Garat S, Gajardo H, Pino AM, Seitz G. Abnormal osteogenesis in osteoporotic patients is reflected by altered mesenchymal stem cells dynamics. J Cell Biochem. 1999. 75:414–423.
Article21. Rodríguez JP, Montecinos L, Ríos S, Reyes P, Martínez J. Mesenchymal stem cells from osteoporotic patients produce a type I collagen-deficient extracellular matrix favoring adipogenic differentiation. J Cell Biochem. 2000. 79:557–565.
Article22. Di Monaco M, Di Monaco R, Manca M, Cavanna A. Positive association between total lymphocyte count and femur bone mineral density in hip-fractured women. Gerontology. 2002. 48:157–161.
Article23. Di Monaco M, Vallero F, Di Monaco R, Mautino F, Cavanna A. Total lymphocyte count and femoral bone mineral density in postmenopausal women. J Bone Miner Metab. 2004. 22:58–63.
Article24. Laudisio A, Marzetti E, Pagano F, Bernabei R, Zuccalá G. Haemoglobin levels are associated with bone mineral density in the elderly: a population-based study. Clin Rheumatol. 2009. 28:145–151.
Article25. Jassal SK, von Muhlen D, Barrett-Connor E. Measures of renal function, BMD, bone loss, and osteoporotic fracture in older adults: the Rancho Bernardo study. J Bone Miner Res. 2007. 22:203–210.
Article26. Manolagas SC. Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev. 2000. 21:115–137.
Article27. Zhai G, Hart DJ, Valdes AM, Kato BS, Richards JB, Hakim A, et al. Natural history and risk factors for bone loss in postmenopausal Caucasian women: a 15-year follow-up population-based study. Osteoporos Int. 2008. 19:1211–1217.
Article28. NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA. 2001. 285:785–795.29. Kim KC, Shin DH, Lee SY, Im JA, Lee DC. Relation between obesity and bone mineral density and vertebral fractures in Korean postmenopausal women. Yonsei Med J. 2010. 51:857–863.
Article30. Chatta GS, Dale DC. Aging and haemopoiesis. Implications for treatment with haemopoietic growth factors. Drugs Aging. 1996. 9:37–47.31. Shin CS, Choi HJ, Kim MJ, Kim JT, Yu SH, Koo BK, et al. Prevalence and risk factors of osteoporosis in Korea: a community-based cohort study with lumbar spine and hip bone mineral density. Bone. 2010. 47:378–387.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The relationship of maturation value of vaginal epithelium and bone mineral density in postmenopausal women
- The relationship between grip strength and femoral and vertebral bone mineral density in peri-and postmenopausal women
- High Serum Osteopontin Levels Are Associated with Low Bone Mineral Density in Postmenopausal Women
- The relationship between grip strength and radius bone mineral density in postmenopausal women
- Influence of the Reproductive Factor and Life Style Factor in Postmenopausal Women's Bone Mineral Density