Yonsei Med J.
2011 Jan;52(1):89-97. 10.3349/ymj.2011.52.1.89.
Clinicopathlogic and Immunohistochemical Characteristics of Triple Negative Invasive Lobular Carcinoma
- Affiliations
-
- 1Department of Pathology, Yonsei University College of Medicine, Seoul, Korea. Jungwh96@yuhs.ac
- KMID: 1106442
- DOI: http://doi.org/10.3349/ymj.2011.52.1.89
Abstract
- PURPOSE
Our study is performed to find out clinicopathlogic and immunohistochemical (IHC) characteristics of triple negative invasive lobular carcinoma (ILC), as has been demonstrated in their invasive ductal counterparts.
MATERIALS AND METHODS
Retrospective analysis of variable clinicopathlogic parameters and IHC stains for androgen receptor, estrogen receptor, progesterone receptor, p53, c-kit, galectin-3, cytokeratin 5 (CK5), CK5/6, vimentin, E-cadherin, epidermal growth factor receptor, and HER2 were performed in 117 cases of ILC.
RESULTS
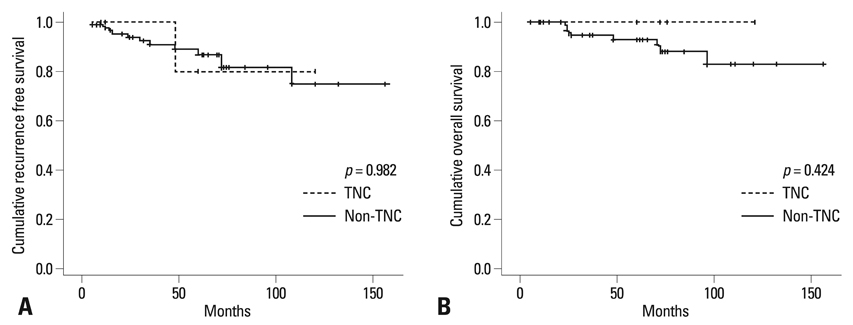
Eight cases (6.8%) were triple negative carcinoma (TNC), which showed higher incidence of high histologic grade than non-TNC (p = 0.019). Galectin-3 was expressed with higher incidence in tumor cells of TNC (62.5%) than those of non-TNC (7.3%) (p = 0.000). In contrast, galectin-3 was expressed with higher incidence in stromal cells of non-TNC (53.2%) than those of TNC (12.5%) (p = 0.029). CK5 and CK5/6 were not expressed in all ILCs.
CONCLUSION
TNC in ILC showed distinct clinicopathologic and IHC characteristics such as higher histologic grade and increased expression of galectin-3, compared to non-TNC in ILC. TNC in ILC was less frequent and did not show CK5 and CK5/6 expression when compared to TNC in invasive ductal carcinoma.
Keyword
MeSH Terms
-
Adult
Breast Neoplasms/*metabolism
Cadherins/metabolism
Carcinoma, Lobular/*metabolism
Female
Galectin 3/metabolism
Humans
Immunohistochemistry/*methods
Keratin-5/metabolism
Keratin-6/metabolism
Middle Aged
Proto-Oncogene Proteins c-kit/metabolism
Receptor, Epidermal Growth Factor/metabolism
Receptors, Androgen
Receptors, Estrogen/metabolism
Receptors, Progesterone/metabolism
Vimentin/metabolism
Figure
Cited by 1 articles
-
Immunophenotypes of Glycogen Rich Clear Cell Carcinoma
Sung Eun Kim, Ja Seung Koo, Woo-Hee Jung
Yonsei Med J. 2012;53(6):1142-1146. doi: 10.3349/ymj.2012.53.6.1142.
Reference
-
1. Arpino G, Bardou VJ, Clark GM, Elledge RM. Infiltrating lobular carcinoma of the breast: tumor characteristics and clinical outcome. Breast Cancer Res. 2004. 6:R149–R156.
Article2. Yeatman TJ, Cantor AB, Smith TJ, Smith SK, Reintgen DS, Miller MS, et al. Tumor biology of infiltrating lobular carcinoma. Implications for management. Ann Surg. 1995. 222:549–559.3. Rakha EA, El-Sayed ME, Powe DG, Green AR, Habashy H, Grainge MJ, et al. Invasive lobular carcinoma of the breast: response to hormonal therapy and outcomes. Eur J Cancer. 2008. 44:73–83.
Article4. Yoder BJ, Wilkinson EJ, Massoll NA. Molecular and morphologic distinctions between infiltrating ductal and lobular carcinoma of the breast. Breast J. 2007. 13:172–179.
Article5. Cleton-Jansen AM. E-cadherin and loss of heterozygosity at chromosome 16 in breast carcinogenesis: different genetic pathways in ductal and lobular breast cancer? Breast Cancer Res. 2002. 4:5–8.
Article6. Sarrió D, Pérez-Mies B, Hardisson D, Moreno-Bueno G, Suárez A, Cano A, et al. Cytoplasmic localization of p120ctn and E-cadherin loss characterize lobular breast carcinoma from preinvasive to metastatic lesions. Oncogene. 2004. 23:3272–3283.
Article7. du Toit RS, Locker AP, Ellis IO, Elston CW, Nicholson RI, Robertson JF, et al. An evaluation of differences in prognosis, recurrence patterns and receptor status between invasive lobular and other invasive carcinomas of the breast. Eur J Surg Oncol. 1991. 17:251–257.8. Tubiana-Hulin M, Stevens D, Lasry S, Guinebretiére JM, Bouita L, Cohen-Solal C, et al. Response to neoadjuvant chemotherapy in lobular and ductal breast carcinomas: a retrospective study on 860 patients from one institution. Ann Oncol. 2006. 17:1228–1233.
Article9. Lamovec J, Bracko M. Metastatic pattern of infiltrating lobular carcinoma of the breast: an autopsy study. J Surg Oncol. 1991. 48:28–33.
Article10. Sastre-Garau X, Jouve M, Asselain B, Vincent-Salomon A, Beuzeboc P, Dorval T, et al. Infiltrating lobular carcinoma of the breast. Clinicopathologic analysis of 975 cases with reference to data on conservative therapy and metastatic patterns. Cancer. 1996. 77:113–120.
Article11. Sørlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001. 98:10869–10874.
Article12. Tischkowitz M, Brunet JS, Bégin LR, Huntsman DG, Cheang MC, Akslen LA, et al. Use of immunohistochemical markers can refine prognosis in triple negative breast cancer. BMC Cancer. 2007. 7:134.
Article13. Livasy CA, Karaca G, Nanda R, Tretiakova MS, Olopade OI, Moore DT, et al. Phenotypic evaluation of the basal-like subtype of invasive breast carcinoma. Mod Pathol. 2006. 19:264–271.
Article14. Nielsen TO, Hsu FD, Jensen K, Cheang M, Karaca G, Hu Z, et al. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res. 2004. 10:5367–5374.
Article15. Cleator S, Heller W, Coombes RC. Triple-negative breast cancer: therapeutic options. Lancet Oncol. 2007. 8:235–244.
Article16. Calza S, Hall P, Auer G, Bjöhle J, Klaar S, Kronenwett U, et al. Intrinsic molecular signature of breast cancer in a population-based cohort of 412 patients. Breast Cancer Res. 2006. 8:R34.
Article17. Jumppanen M, Gruvberger-Saal S, Kauraniemi P, Tanner M, Bendahl PO, Lundin M, et al. Basal-like phenotype is not associated with patient survival in estrogen-receptor-negative breast cancers. Breast Cancer Res. 2007. 9:R16.
Article18. Rouzier R, Perou CM, Symmans WF, Ibrahim N, Cristofanilli M, Anderson K, et al. Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin Cancer Res. 2005. 11:5678–5685.
Article19. Sotiriou C, Neo SY, McShane LM, Korn EL, Long PM, Jazaeri A, et al. Breast cancer classification and prognosis based on gene expression profiles from a population-based study. Proc Natl Acad Sci U S A. 2003. 100:10393–10398.
Article20. Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000. 406:747–752.
Article21. van 't Veer LJ, Dai H, van de Vijver MJ, He YD, Hart AA, Mao M, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002. 415:530–536.22. Abd El-Rehim DM, Pinder SE, Paish CE, Bell J, Blamey RW, Robertson JF, et al. Expression of luminal and basal cytokeratins in human breast carcinoma. J Pathol. 2004. 203:661–671.
Article23. Piekarski JH, Biernat W. Clinical significance of CK5/6 and PTEN protein expression in patients with bilateral breast carcinoma. Histopathology. 2006. 49:248–255.
Article24. Reis-Filho JS, Simpson PT, Martins A, Preto A, Gärtner F, Schmitt FC. Distribution of p63, cytokeratins 5/6 and cytokeratin 14 in 51 normal and 400 neoplastic human tissue samples using TARP-4 multi-tumor tissue microarray. Virchows Arch. 2003. 443:122–132.
Article25. Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 2002. 41:154–161.
Article26. Cutler SJ, Black MM, Mork T, Harvei S, Freeman C. Further observations on prognostic factors in cancer of the female breast. Cancer. 1969. 24:653–667.
Article27. Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 1999. 17:1474–1481.
Article28. Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007. 25:118–145.
Article29. Barondes SH, Cooper DN, Gitt MA, Leffler H. Galectins. Structure and function of a large family of animal lectins. J Biol Chem. 1994. 269:20807–20810.
Article30. Bao Q, Hughes RC. Galectin-3 expression and effects on cyst enlargement and tubulogenesis in kidney epithelial MDCK cells cultured in three-dimensional matrices in vitro. J Cell Sci. 1995. 108:2791–2800.
Article31. Dagher SF, Wang JL, Patterson RJ. Identification of galectin-3 as a factor in pre-mRNA splicing. Proc Natl Acad Sci U S A. 1995. 92:1213–1217.
Article32. Le Marer N, Hughes RC. Effects of the carbohydrate-binding protein galectin-3 on the invasiveness of human breast carcinoma cells. J Cell Physiol. 1996. 168:51–58.
Article33. Sato S, Hughes RC. Binding specificity of a baby hamster kidney lectin for H type I and II chains, polylactosamine glycans, and appropriately glycosylated forms of laminin and fibronectin. J Biol Chem. 1992. 267:6983–6990.
Article34. Xu XC, el-Naggar AK, Lotan R. Differential expression of galectin-1 and galectin-3 in thyroid tumors. Potential diagnostic implications. Am J Pathol. 1995. 147:815–822.35. Lotan R, Ito H, Yasui W, Yokozaki H, Lotan D, Tahara E. Expression of a 31-kDa lactoside-binding lectin in normal human gastric mucosa and in primary and metastatic gastric carcinomas. Int J Cancer. 1994. 56:474–480.
Article36. Schoeppner HL, Raz A, Ho SB, Bresalier RS. Expression of an endogenous galactose-binding lectin correlates with neoplastic progression in the colon. Cancer. 1995. 75:2818–2826.
Article37. Castronovo V, Van Den Brûle FA, Jackers P, Clausse N, Liu FT, Gillet C, et al. Decreased expression of galectin-3 is associated with progression of human breast cancer. J Pathol. 1996. 179:43–48.
Article38. Moon BK, Lee YJ, Battle P, Jessup JM, Raz A, Kim HR. Galectin-3 protects human breast carcinoma cells against nitric oxide-induced apoptosis: implication of galectin-3 function during metastasis. Am J Pathol. 2001. 159:1055–1060.
Article39. Moisa A, Fritz P, Eck A, Wehner HD, Mürdter T, Simon W, et al. Growth/adhesion-regulatory tissue lectin galectin-3: stromal presence but not cytoplasmic/nuclear expression in tumor cells as a negative prognostic factor in breast cancer. Anticancer Res. 2007. 27:2131–2139.40. Shekhar MP, Nangia-Makker P, Tait L, Miller F, Raz A. Alterations in galectin-3 expression and distribution correlate with breast cancer progression: functional analysis of galectin-3 in breast epithelial-endothelial interactions. Am J Pathol. 2004. 165:1931–1941.
Article41. Fadare O, Tavassoli FA. The phenotypic spectrum of basal-like breast cancers: a critical appraisal. Adv Anat Pathol. 2007. 14:358–373.
Article42. Fadare O, Tavassoli FA. Clinical and pathologic aspects of basal-like breast cancers. Nat Clin Pract Oncol. 2008. 5:149–159.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Nodular Metastatic Carcinoma from Invasive Lobular Breast Cancer
- Invasive Lobular Carcinoma of the Breast Associated with Mixed Lobular and Ductal Carcinoma In Situ: A Case Report
- Clinical Analysis of an Invasive Lobular Carcinoma in the Breast
- Lobular carcinoma in situ in sclerosing adenosis
- Invasive Lobular Carcinoma Mimicking Fat Necrosis in Breast: A Case Report