Korean J Ophthalmol.
2010 Feb;24(1):47-52. 10.3341/kjo.2010.24.1.47.
Subconjunctival Bevacizumab as an Adjunct to Trabeculectomy in Eyes with Refractory Glaucoma: A Case Series
- Affiliations
-
- 1HanGil Eye Hospital, Incheon, Korea. deskshot@naver.com
- KMID: 1098794
- DOI: http://doi.org/10.3341/kjo.2010.24.1.47
Abstract
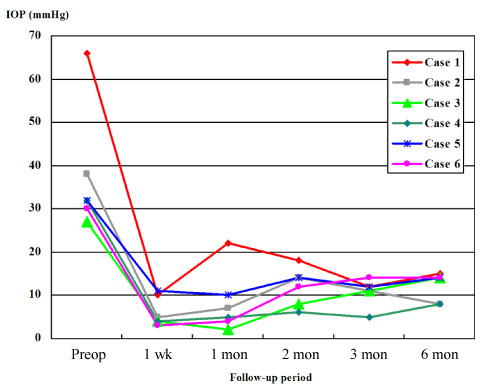
- This prospective observational case series study included 6 eyes of 6 consecutive glaucomatous patients. Each patient underwent trabeculectomy with mitomycin C, and received a 1.25 mg of subconjunctival bevacizumab injection at completion of the trabeculectomy. Study eyes included two with neovascular glaucoma, three with uveitic glaucoma, and one with secondary glaucoma following vitrectomy. All eyes had undergone failed glaucoma laser/surgical treatment or an intraocular surgical procedure. Intraocular pressure (IOP) at the following postoperative visits: preoperative, 1 week, 1 month, 2 months, 3 months, and 6 months, was measured. We also evaluated postoperative bleb findings and complications. IOP measured at each visit was 37.5+/-14.4 mmHg, 6.2+/-3.4 mmHg, 8.3+/-7.2 mmHg, 12.0+/-4.4 mmHg, 10.8+/-3.1 mmHg, and 12.2+/-3.3 mmHg, respectively, for each visit. All eyes had functioning blebs with normal IOP at postoperative 6 months with no additional IOP-lowering medication.
Keyword
MeSH Terms
-
Adult
Aged
Angiogenesis Inhibitors/*administration & dosage
Antibodies, Monoclonal/*administration & dosage
Conjunctiva
Female
Glaucoma/*drug therapy/etiology/*surgery
Glaucoma, Neovascular/drug therapy/surgery
Humans
Injections, Intraocular
Male
Middle Aged
Prospective Studies
Trabeculectomy/*methods
Uveitis/complications
Vascular Endothelial Growth Factor A/*antagonists & inhibitors
Vitrectomy/adverse effects
Figure
Reference
-
1. Gan L, Fagerholm P, Palmblad J. Vascular endothelial growth factor (VEGF) and its receptor VEGFR-2 in the regulation of corneal neovascularization and wound healing. Acta Ophthalmol Scand. 2004. 82:557–563.2. Presta LG, Chen H, O'Connor SJ, et al. Humanization of an anti-vascular endothelial growth factor monoclonal antibody for the therapy of solid tumors and other disorders. Cancer Res. 1997. 57:4593–4599.3. Miller KD, Chap LI, Holmes FA, et al. Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J Clin Oncol. 2005. 23:792–799.4. Rich RM, Rosenfeld PJ, Puliafito CA, et al. Short-term safety and efficacy of intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Retina. 2006. 26:495–511.5. Avery RL, Pearlman J, Pieramici DJ, et al. Intravitreal bevacizumab (Avastin) in the treatment of proliferative diabetic retinopathy. Ophthalmology. 2006. 113:1695.e1–1695.e15.6. Wakabayashi T, Oshima Y, Sakaguchi H, et al. Intravitreal bevacizumab to treat iris neovascularization and neovascular glaucoma secondary to ischemic retinal diseases in 41 consecutive cases. Ophthalmology. 2008. 115:1571–1580.7. Manzano RP, Peyman GA, Khan P, et al. Inhibition of experimental corneal neovascularisation by bevacizumab (Avastin). Br J Ophthalmol. 2007. 91:804–807.8. Kim SW, Ha BJ, Kim EK, et al. The effect of topical bevacizumab on corneal neovascularization. Ophthalmology. 2008. 115:e33–e38.9. Wilgus TA, Ferreira AM, Oberyszyn TM, et al. Regulation of scar formation by vascular endothelial growth factor. Lab Invest. 2008. 88:579–590.10. Li Z, Van Bergen T, Van de Veire S, et al. Inhibition of vascular endothelial growth factor reduces scar formation after glaucoma filtration surgery. Invest Ophthalmol Vis Sci. 2009. 50:5217–5225.11. Jonas JB, Spandau UH, Schlichtenbrede F. Intravitreal bevacizumab for filtering surgery. Ophthalmic Res. 2007. 39:121–122.12. Grewal DS, Jain R, Kumar H, Grewal SP. Evaluation of subconjunctival bevacizumab as an adjunct to trabeculectomy: a pilot study. Ophthalmology. 2008. 115:2141–2145.13. Lama PJ, Fechtner RD. Antifibrotics and wound healing in glaucoma surgery. Surv Ophthalmol. 2003. 48:314–346.14. Jordan JF, Diestelhorst M, Grisanti S, Krieglstein GK. Photodynamic modulation of wound healing in glaucoma filtration surgery. Br J Ophthalmol. 2003. 87:870–875.15. Lu DW, Chang CJ, Chiang CH, et al. Wound modulation after trabeculectomy by different formulations of antimetabolites in rabbits. J Ocul Pharmacol Ther. 2000. 16:529–538.16. Demir T, Turgut B, Celiker U, et al. Effects of octreotide acetate and amniotic membrane on wound healing in experimental glaucoma surgery. Doc Ophthalmol. 2003. 107:87–92.17. Chaudhary NI, Roth GJ, Hilberg F, et al. Inhibition of PDGF, VEGF and FGF signalling attenuates fibrosis. Eur Respir J. 2007. 29:976–985.18. Vote B, Fuller JR, Bevin TH, Molteno AC. Systemic anti-inflammatory fibrosis suppression in threatened trabeculectomy failure. Clin Experiment Ophthalmol. 2004. 32:81–86.19. Bock F, Onderka J, Dietrich T, et al. Bevacizumab as a potent inhibitor of inflammatory corneal angiogenesis and lymphangiogenesis. Invest Ophthalmol Vis Sci. 2007. 48:2545–2552.20. Wu WS, Wang FS, Yang KD, et al. Dexamethasone induction of keloid regression through effective suppression of VEGF expression and keloid fibroblast proliferation. J Invest Dermatol. 2006. 126:1264–1271.21. Zhang N, Fang Z, Contag PR, et al. Tracking angiogenesis induced by skin wounding and contact hypersensitivity using a Vegfr2-luciferase transgenic mouse. Blood. 2004. 103:617–626.22. Memarzadeh F, Varma R, Lin LT, et al. Postoperative use of bevacizumab as an antifibrotic agent in glaucoma filtration surgery in the rabbit. Invest Ophthalmol Vis Sci. 2009. 50:3233–3237.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Two Cases of Subconjunctival Bevacizumab Injection to Prevent Bleb Failure After Trabeculectomy
- Clinical Results of Trabeculectomy
- A Clinical Evaluations of Trabeculectomy
- Trabeculectomy with Adjunctive Mitomycin-C: Compariative Study of Subconjunctival Injection and Soaking of Mitomycin-C (I)
- A Clinical Evaluations of Trabeculectomy