Korean J Lab Med.
2010 Feb;30(1):9-16. 10.3343/kjlm.2010.30.1.9.
Evaluation of Analytical Measurement Ranges of Three Full Range C-Reactive Protein Tests Using Immunoturbidimetric Assay
- Affiliations
-
- 1Department of Laboratory Medicine, School of Medicine, Wonkwang University, Iksan, Korea. email@wku.ac.kr
- 2Institute of Wonkwang Medical Science, School of Medicine, Wonkwang University, Iksan, Korea.
- KMID: 1096812
- DOI: http://doi.org/10.3343/kjlm.2010.30.1.9
Abstract
- BACKGROUND
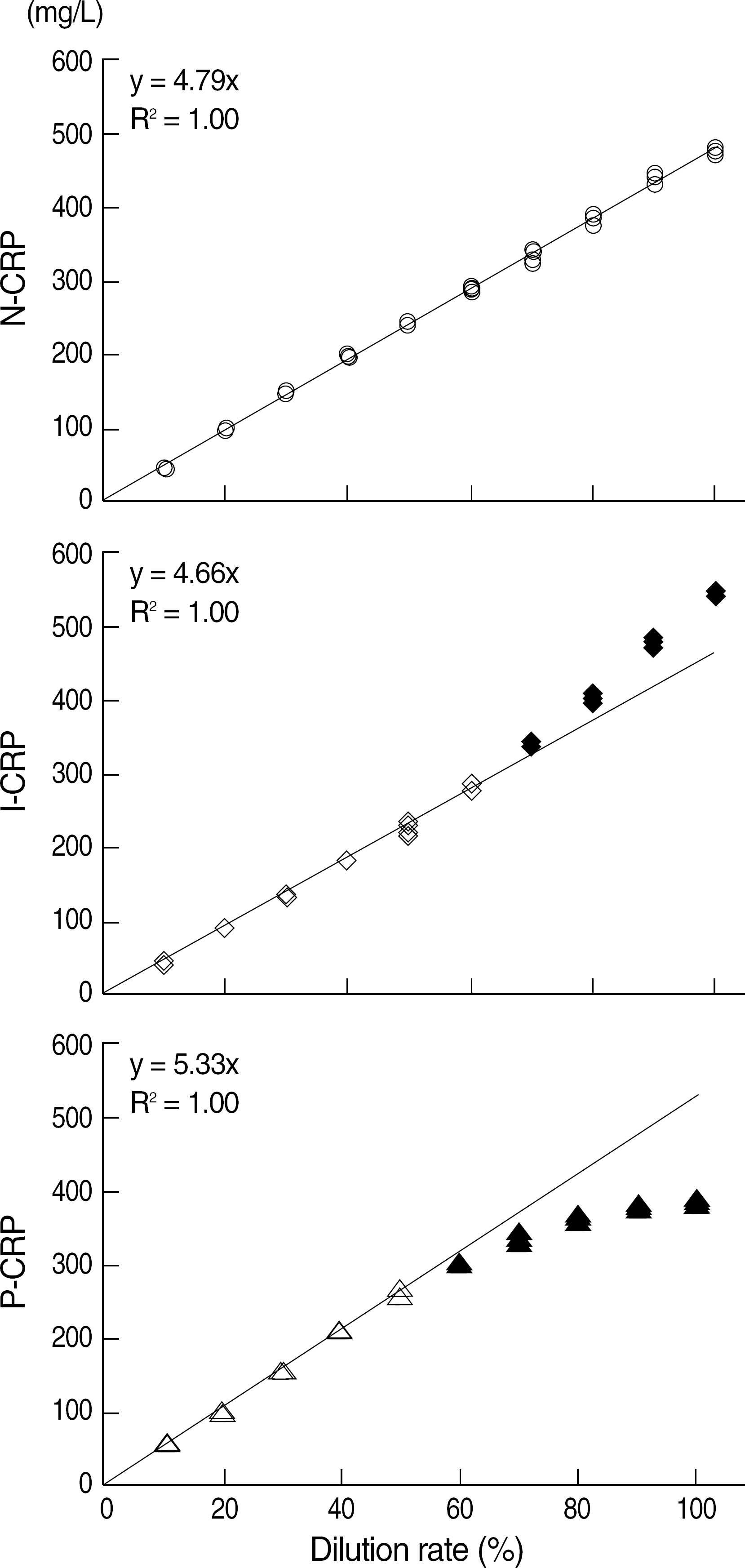
Recently developed full-range C-reactive protein (CRP) tests, which are based on the immunoturbidimetric method, have wider analytical measurement ranges (AMR) than previously used tests. We evaluated the AMR of 3 full-range CRP tests-2 new and 1 previously used test.
METHODS
We analyzed the precision and AMR of 2 full-range CRP tests (Sekisui, Nanopia CRP, N-CRP and Iatron, IATRO CRP-EX, I-CRP) and compared the values obtained for these tests with those obtained for the conventional full-range CRP test (Sekisui, PureAuto S CRP, P-CRP). We evaluated the tests for the limit of quantification and for linearity. We also compared these results of these tests by using the comparative test (Dade Behring, cCRP) for cardiovascular risk assessment.
RESULTS
Coefficients of variation (CVs) of all the full-range CRP tests were less than 10% for concentrations greater than 0.6 mg/L, and CVs of N-CRP and I-CRP were lower than those of P-CRP for concentrations less than 1 mg/L. N-CRP (0.1-467 mg/L) and I-CRP (0.1-280 mg/L) had wider AMR than P-CRP (3-233 mg/L). All the full-range CRP tests showed more than 90% agreement with the cCRP values for the assessment of cardiovascular risk.
CONCLUSIONS
The 3 full-range CRP tests, by virtue of their wide AMR, may be used for the detection of acute inflammation as well as for the assessment of cardiovascular risk. N-CRP and I-CRP may be more useful than P-CRP for determining the CRP concentration, especially for the detection of concentrations close to the lower or upper limit of the analytical range, without the need for repetition of the test.
MeSH Terms
Figure
Reference
-
1.Pearson TA., Mensah GA., Alexander RW., Anderson JL., Cannon RO 3rd., Criqui M, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003. 107:499–511.2.Roberts WL., Sedrick R., Moulton L., Spencer A., Rifai N. Evaluation of four automated high-sensitivity C-reactive protein methods: implications for clinical and epidemiological applications. Clin Chem. 2000. 46:461–8.
Article3.US Food and Drug Administration. Guidance for industry and FDA staff: Review criteria for assessment of C-reactive protein (CRP), high sensitivity C-reactive protein (hsCRP), and cardiac C-reactive protein (cCRP) assays. Silver Spring, MD: U.S. Center for Devices and Radiological Health. 2005.4.Kang SY., Suh JT., Kim JH., Lee WI., Lee HJ. Comparison of high sensitivity C-reactive protein assay with a wide assay range. Korean J Lab Med. 2005. 25:227–33. (강소영, 서진태, 김정훈, 이우인, 이희주. 측정범위가 넓은 C-Reactive Protein 검사법 비교. 대한진단검사의학회지 2005;25:227-33.).5.Sánchez A., Mirabel JL., Barrenechea E., Eugui J., Puelles A., Castañeda A. Evaluation of an improved immunoturbidimetic assay for serum C-reactive protein on a COBAS INTEGRA 400 Analyzer. Clin Lab. 2002. 48:313–7.6.Jang SS., Lee W., Chun S., Min WK. Evaluation of cobas integra 700 and distribution of high sensitivity C-reactive protein levels in Koreans. Korean J Lab Med. 2002. 22:319–24. (장성수, 이우창, 전사일, 민원기. 자동화학분석기 Cobas Integra 700의 평가 및 High Sensitivity CReactiveProtein의참고치분포. 대한진단검사의학회지 2002;22:319-24.).7.Piéroni L., Chahbani N., Hainque B. Verification of the analytical range of a new reagent for full-range C-reactive protein determination. Clin Chem Lab Med. 2009. 47:109–11.
Article8.Marie Dupuy A., Boutet A., Paul Cristol J. Evaluation of the high-sensitivity, full-range Olympus CRP OSR6199 application on the Olympus AU640. Clin Chem Lab Med. 2007. 45:402–6.
Article9.Clinical and Laboratory Standards Institute. Evaluation of precision performance of quantitative measurement methods; approved guideline. CLSI document EP5-A2. Wayne, PA: CLSI;2004.10.Armbruster DA., Pry T. Limit of blank, limit of detection and limit of quantitation. Clin Biochem Rev. 2008. 29(S1):49–52.11.Clinical and Laboratory Standards Institute. Evaluation of the linearity of quantitative measurement procedures: a statistical approach; approved guideline. CLSI document EP6-A. Wayne, PA: CLSI;2003.12.Emancipator K., Kroll MH. A quantitative measure of nonlinearity. Clin Chem. 1993. 39:766–72.
Article13.Bland JM., Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986. 1:307–10.
Article14.Ridker PM., Danielson E., Fonseca FA., Genest J., Gotto AM Jr., Kastelein JJ, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008. 359:2195–207.
Article15.Mora S., Ridker PM. Justification for the Use of Statins in Primary Prevention: an Intervention Trial Evaluating Rosuvastatin (JUP-ITER)—can C-reactive protein be used to target statin therapy in primary prevention? Am J Cardiol. 2006. 97:33A–41A.
Article16.Nguyen XM., Lane J., Smith BR., Nguyen NT. Changes in inflammatory biomarkers across weight classes in a representative US population: a link between obesity and inflammation. J Gastrointest Surg. 2009. 13:1205–12.
Article17.Kelley DS., Siegel D., Fedor DM., Adkins Y., Mackey BE. DHA supplementation decreases serum C-reactive protein and other markers of inflammation in hypertriglyceridemic men. J Nutr. 2009. 139:495–501.
Article18.Johnson AM. A new international reference preparation for proteins in human serum. Arch Pathol Lab Med. 1993. 117:29–31.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Development and Evaluation of New Immunoturbidimetric Reagents for Apo A-I and B in the Human Serum
- Analytical Performance of INNOVANCE Free Protein S Antigen on Sysmex CS-5100
- Reference Range of HE4 in Healthy Women: Analytical Performance and Correlation with CA125
- Usefulness of plasma interleukin-6 and C-reactive protein levels in differential diagnosis of clonal and reactive thrombocytosis
- Analytical Performance of New ARCHITECT AFP Assay: Comparison with the Current Assay