Korean J Lab Med.
2010 Apr;30(2):111-116. 10.3343/kjlm.2010.30.2.111.
Molecular Analysis of Two Cases of Severe Congenital Neutropenia
- Affiliations
-
- 1Department of Laboratory Medicine, College of Medicine, The Catholic University of Korea, Seoul, Korea. hankja@catholic.ac.kr
- 2Department of Pediatrics, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- KMID: 1096794
- DOI: http://doi.org/10.3343/kjlm.2010.30.2.111
Abstract
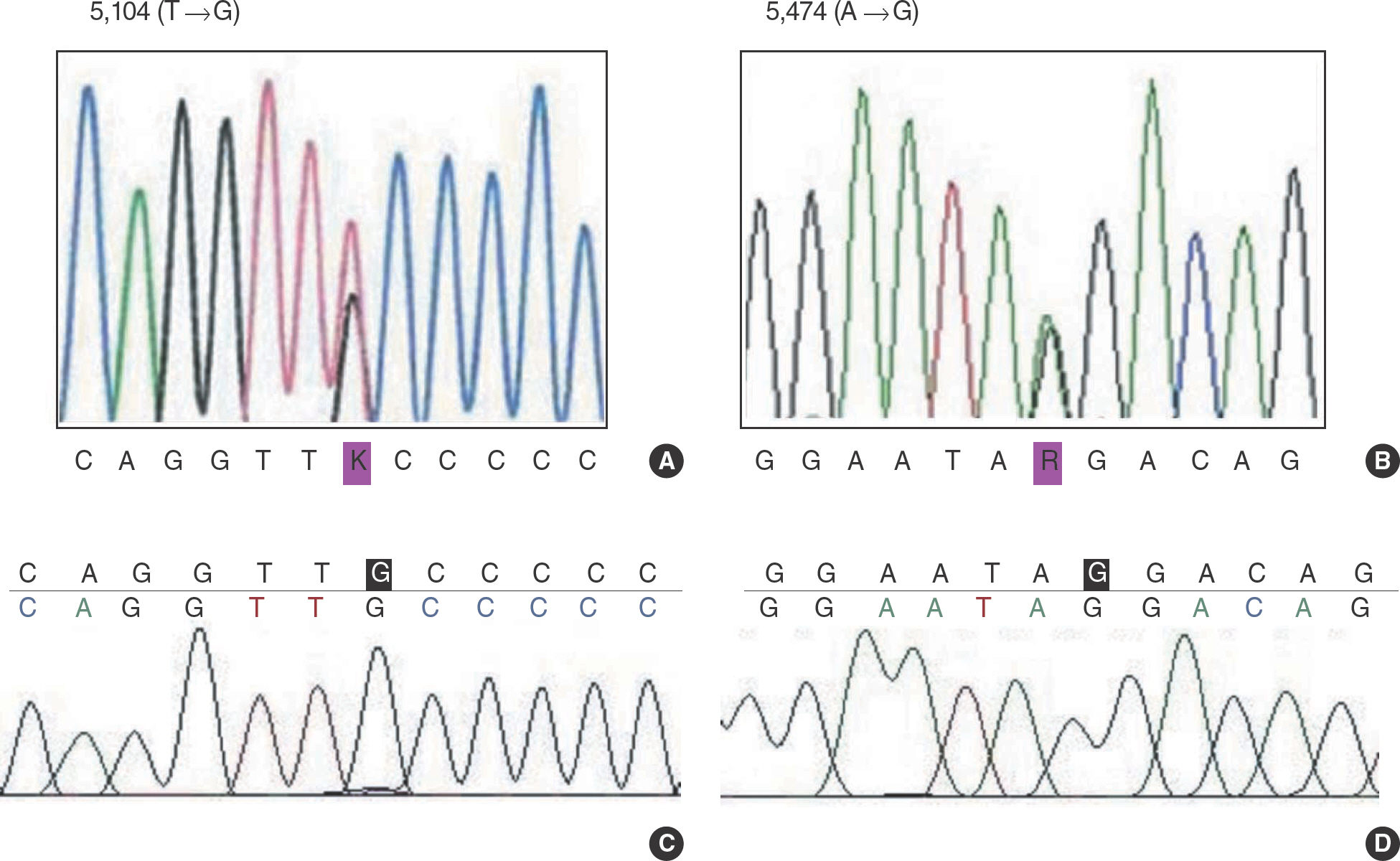
- Severe congenital neutropenia is a rare hematological disease characterized by a selective decrease in circulating neutrophils, maturation arrest of granulocytic precursors at the promyelocyte stage, and recurrence of infections. A 2-month-old male infant (patient A) and a 14-month-old female child (patient B) were referred to our hospital due to severe neutropenia. Sequencing analysis of ELA2 and HAX1 genes was performed. Two single nucleotide polymorphisms of HAX1 gene were found. They were 5,104T-->G point mutation of exon 1 and 5,474A-->G point mutation of intron 1 in HAX1 gene. The mutation of ELA2 gene was not found. The patient A showed a good response to granulocyte colony-stimulating factor (G-CSF) treatment and the absolute neutrophil count recovered to 1,195/microliter. But the patient B showed a partial response to G-CSF treatment and experienced several episodes of herpetic gingivostomatitis, oral ulcer, acute pharyngotonsillitis and otitis media during follow-up.
Keyword
MeSH Terms
-
Adaptor Proteins, Signal Transducing/genetics
Bone Marrow/pathology
Female
Granulocyte Colony Stimulating Factor, Recombinant/adverse effects/therapeutic use
Humans
Infant
Male
Neutropenia/congenital/drug therapy/*genetics
Neutrophils/cytology/pathology
Oral Ulcer/etiology
Otitis Media/etiology
Polymorphism, Single Nucleotide
Serine Endopeptidases/genetics
Stomatitis, Herpetic/etiology
Figure
Reference
-
1.Boxer LA. Severe congenital neutropenia: genetics and pathogenesis. Trans Am Clin Climatol Assoc. 2006. 117:13–31.2.Kostmann R. Infantile genetic agranulocytosis. A review with presentation of ten new cases. Acta Paediatr Scand. 1975. 64:362–8.3.Dale DC., Person RE., Bolyard AA., Aprikyan AG., Bos C., Bonilla MA, et al. Mutations in the gene encoding neutrophil elastase in congenital and cyclic neutropenia. Blood. 2000. 96:2317–22.
Article4.Horwitz MS., Duan Z., Korkmaz B., Lee HH., Mealiffe ME., Salipante SJ. Neutrophil elastase in cyclic and severe congenital neutropenia. Blood. 2007. 109:1817–24.
Article5.Klein C., Grudzien M., Appaswamy G., Germeshausen M., Sandrock I., Schaffer AA, et al. HAX1 deficiency causes autosomal recessive severe congenital neutropenia (Kostmann disease). Nat Genet. 2007. 39:86–92.
Article6.Germeshausen M., Grudzien M., Zeidler C., Abdollahpour H., Yetgin S., Rezaei N, et al. Novel HAX1 mutations in patients with severe congenital neutropenia reveal isoform-dependent genotype-phenotype associations. Blood. 2008. 111:4954–7.7.Kawaguchi H., Kobayashi M., Nakamura K., Konishi N., Miyagawa S., Sato T, et al. Dysregulation of transcriptions in primary granule constituents during myeloid proliferation and differentiation in patients with severe congenital neutropenia. J Leukoc Biol. 2003. 73:225–34.
Article8.Ishikawa N., Okada S., Miki M., Shirao K., Kihara H., Tsumura M, et al. Neurodevelopmental abnormalities associated with severe congenital neutropenia due to the R86X mutation in the HAX1 gene. J Med Genet. 2008. 45:802–7.9.Kostmann R. Infantile genetic agranulocytosis (agranulocytosis infantilis hereditaria): a new recessive lethal disease in man. Acta Pediatr Scand. 1956. 45:1–78.
Article10.Boxer L., Dale DC. Neutropenia: causes and consequences. Semin Hematol. 2002. 39:75–81.
Article11.Freedman MH., Bonilla MA., Fier C., Bolyard AA., Scarlata D., Boxer LA, et al. Myelodysplasia syndrome and acute myeloid leukemia in patients with congenital neutropenia receiving G-CSF therapy. Blood. 2000. 96:429–36.12.Freedman MH., Alter BP. Risk of myelodysplastic syndrome and acute myeloid leukemia in congenital neutropenias. Semin Hematol. 2002. 39:128–33.
Article13.Yoo HW., Shin SM., Ahn HS., Choi Y., Hong CY. A case of Kostmann syndrome. J Korean Pediatr Soc. 1983. 26:284–8. (유한욱, 신손문, 안효섭, 최용, 홍창의. Kostmann 증후군1례. 소아과 1983;26:284-8.).14.Shin WS., Kim SW., Paik IK. A case of Kostmann syndrome. J Korean Pediatr Soc. 1989. 32:1568–73. (신원섭, 김상우, 백인기. Kostmann 증후군1례. 소아과 1989;32:1568-73.).15.Kim SC., Kim MY., Song KE., Suh JS., Lee WK., Kim JS. A case of congenital neutropenia. Korean J Clin Pathol. 1989. 9:385–9. (김성철, 김문연, 송경은, 서장수, 이원길, 김재식. 선천성 호중구 감소증 1예. 대한임상병리학회지 1989;9:385-9.).16.Yoon CG., Jang JG., Sin JB., Lee SY. Kostmann's disease: a case report. Korean J Pediatr Hematol-Oncol. 1999. 6:136–40. (윤철규, 장진규, 신종범, 이순용. Kostmann병. 대한소아혈액종양학회지 1999;6:136-40.).17.Lee JH., Cho B., Sung IK., Kim HK., Lee KS., Han KJ. A case of Kostmann syndrome treated with recombinant human granulocyte colony-stimulating factor. Korean J Perinatol. 1996. 7:188–93. (이정화, 조 빈, 성인경, 김학기, 이경수, 한경자. 과립구집락자극인자로치료한 Kostmann 증후군1례. 대한주산의학회잡지 1996;7:188-93.).18.Dale DC., Bonilla MA., Davis MW., Nakanishi AM., Hammond WP., Kurtzberg J, et al. A randomized controlled phase III trial of recombinant human granulocyte colony-stimulating factor (filgrastim) for treatment of severe chronic neutropenia. Blood. 1993. 81:2496–502.
Article19.Freedman MH. Safety of long-term administration of granulocyte colony-stimulating factor for severe chronic neutropenia. Curr Opin Hematol. 1997. 4:217–24.
Article20.Welte K., Dale D. Pathophysiology and treatment of severe chronic neutropenia. Ann Hematol. 1996. 72:158–65.
Article21.Welte K., Boxer LA. Severe chronic neutropenia: pathophysiology and therapy. Semin Hematol. 1997. 34:267–78.22.Welte K., Zeidler C., Dale DC. Severe congenital neutropenia. Semin Hematol. 2006. 43:189–95.
Article23.Zeidler C., Welte K., Barak Y., Barriga F., Bolyard AA., Boxer L, et al. Stem cell transplantation in patients with severe congenital neutropenia without evidence of leukemic transformation. Blood. 2000. 95:1195–8.24.Seo JJ., Moon SH., Cha SW., Ko KO., Chung YH., Anh HS, et al. Effect of granulocyte colony-stimulating factor in a patient with congenital agranulocytosis. Korean J BRM. 1994. 4:123–30. (서종진, 문신혜, 차상원, 고경옥, 정용헌, 안효섭 외. 선천성 무과립구증에 대한 과립구 집락자극인자(G-CSF)의치료. 한국BRM 학회지 1994;4:123-30.).25.Ancliff PJ., Gale RE., Liesner R., Hann IM., Linch DC. Mutations in the ELA2 gene encoding neutrophil elastase are present in most patients with sporadic severe congenital neutropenia but only in some patients with the familial form of the disease. Blood. 2001. 98:2645–50.26.Dong F., Brynes RK., Tidow N., Welte K., Lowenberg B., Touw IP. Mutations in the gene for the granulocyte colony-stimulating-factor receptor in patients with acute myeloid leukemia preceded by severe congenital neutropenia. N Engl J Med. 1995. 333:487–93.
Article27.Devriendt K., Kim AS., Mathijs G., Frints SG., Schwartz M., Van Den Oord JJ, et al. Constitutively activating mutation in WASP causes X-linked severe congenital neutropenia. Nat Genet. 2001. 27:313–7.
Article28.Person RE., Li FQ., Duan Z., Benson KF., Wechsler J., Papadaki HA, et al. Mutations in proto-oncogene GFI1 cause human neutropenia and target ELA2. Nat Genet. 2003. 34:308–12.29.Skokowa J., Cario G., Uenalan M., Schambach A., Germeshausen M., Battmer K, et al. LEF-1 is crucial for neutrophil granulocytopoiesis and its expression is severely reduced in congenital neutropenia. Nat Med. 2006. 12:1191–7.
Article30.Lee ST., Yoon HS., Kim HJ., Lee JH., Park JH., Kim SH, et al. A novel mutation Ala57Val of the ELA2 gene in a Korean boy with severe congenital neutropenia. Ann Hematol. 2009. 88:593–5.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Different Clinical Phenotypes in Familial Severe Congenital Neutropenia Cases with Same Mutation of the ELANE Gene
- A Case of Atypical Kawasaki Disease with Severe Neutropenia
- Neutropenia in children
- Improvement of neutropenia in 2 adolescent cases treated with empagliflozin for glycogen storage disease type Ib
- Clinical Characteristics of Severe Neutropenia in Children