Pediatr Emerg Med J.
2024 Jul;11(3):136-141. 10.22470/pemj.2024.01011.
Improvement of neutropenia in 2 adolescent cases treated with empagliflozin for glycogen storage disease type Ib
- Affiliations
-
- 1Department of Pediatrics, Kyungpook National University Chilgok Hospital, Daegu, Republic of Korea
- KMID: 2557052
- DOI: http://doi.org/10.22470/pemj.2024.01011
Abstract
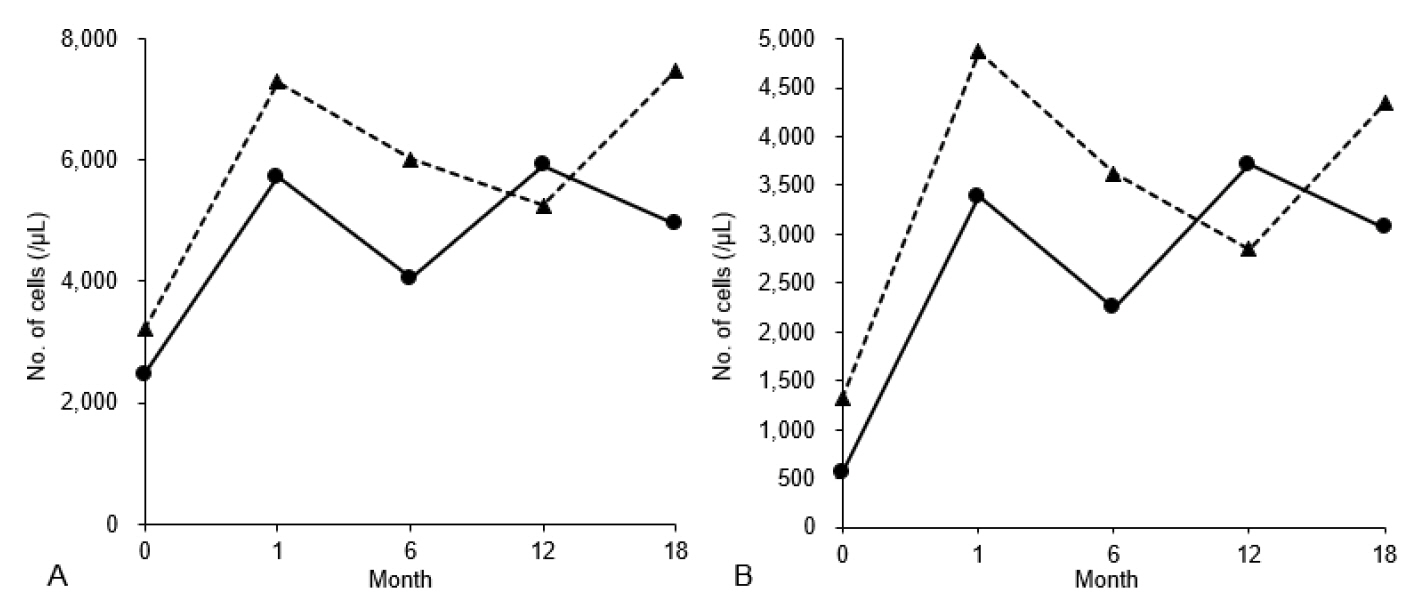
- Neutropenia and elevated concentrations of hepatic transaminases often lead to referrals from emergency or outpatient departments to relevant specialists to diagnose underlying inborn errors of metabolism. Glycogen storage disease type (GSD) Ib, a rare congenital disorder of glucose metabolism caused by the SLC37A4 gene mutations, shows various manifestations, including persistent neutropenia and elevated hepatic transaminases. Empagliflozin has demonstrated its efficacy in treating GSD Ib-associated neutropenia by reducing the entry of 1,5-anhydroglucitol-6-phosphate into the neutrophils. This article reports successful empagliflozin therapy for GSD Ib-related neutropenia in 2 Korean adolescents. Diagnosing GSD Ib is complex and usually initiated by a referral from emergency or outpatient departments when there is a high index of suspicion. Once diagnosed, empagliflozin shows promising outcomes in restoring counts and function of the neutrophils without severe adverse effects in children with GSD Ib, supporting it as a safe and effective therapeutic option for GSD Ib-associated neutropenia.
Keyword
Figure
Reference
-
References
1. Pascual C, Trenchs V, Hernandez-Bou S, Catala A, Valls AF, Luaces C. Outcomes and infectious etiologies of febrile neutropenia in non-immunocompromised children who present in an emergency department. Eur J Clin Microbiol Infect Dis. 2016; 35:1667–72.
Article2. Thomas AE, Simpson LA. A step-by-step approach to paediatric neutropenia. Paediatr Child Health. 2017; 27:511–6.
Article3. van Schaftingen E, Gerin I. The glucose-6-phosphatase system. Biochem J. 2002; 362:513–32.
Article4. Veiga-da-Cunha M, Chevalier N, Stephenne X, Defour JP, Paczia N, Ferster A, et al. Failure to eliminate a phosphorylated glucose analog leads to neutropenia in patients with G6PT and G6PC3 deficiency. Proc Natl Acad Sci U S A. 2019; 116:1241–50.
Article5. Halligan R, White FJ, Schwahn B, Stepien KM, Kamarus Jaman N, McSweeney M, et al. The natural history of glycogen storage disease type Ib in England: a multisite survey. JIMD Rep. 2021; 59:52–9.
Article6. Grunert SC, Derks TGJ, Adrian K, Al-Thihli K, Ballhausen D, Bidiuk J, et al. Efficacy and safety of empagliflozin in glycogen storage disease type Ib: data from an international questionnaire. Genet Med. 2022; 24:1781–8.
Article7. Bidiuk J, Gaciong ZA, Sobieraj P. The overall benefits of empagliflozin treatment in adult siblings with glycogen storage disease type Ib: one year experience. Arch Med Sci. 2022; 18:1095–9.
Article8. Rossi A, Miele E, Fecarotta S, Veiga-da-Cunha M, Martinelli M, Mollica C, et al. Crohn disease-like enterocolitis remission after empagliflozin treatment in a child with glycogen storage disease type Ib: a case report. Ital J Pediatr. 2021; 47:149.
Article9. Grunert SC, Elling R, Maag B, Wortmann SB, Derks TGJ, Hannibal L, et al. Improved inflammatory bowel disease, wound healing and normal oxidative burst under treatment with empagliflozin in glycogen storage disease type Ib. Orphanet J Rare Dis. 2020; 15:218.
Article10. Hexner-Erlichman Z, Veiga-da-Cunha M, Zehavi Y, Vadasz Z, Sabag AD, Tatour S, et al. Favorable outcome of empagliflozin treatment in two pediatric glycogen storage disease type 1b patients. Front Pediatr. 2022; 10:1071464.
Article11. Kelley J, Cooling L. Evidence for adverse effects by G-CSF in some acute lymphoblastic leukemias. Transfus Apher Sci. 2023; 62:103637.
Article12. Lapidari P, Vaz-Luis I, Di Meglio A. Side effects of using granulocyte-colony stimulating factors as prophylaxis of febrile neutropenia in cancer patients: a systematic review. Crit Rev Oncol Hematol. 2021; 157:103193.
Article13. Wortmann SB, Van Hove JLK, Derks TGJ, Chevalier N, Knight V, Koller A, et al. Treating neutropenia and neutrophil dysfunction in glycogen storage disease type Ib with an SGLT2 inhibitor. Blood. 2020; 136:1033–43.
Article14. Beyzaei Z, Geramizadeh B. Molecular diagnosis of glycogen storage disease type I: a review. EXCLI J. 2019; 18:30–46.15. Han SH, Ki CS, Lee JE, Hong YJ, Son BK, Lee KH, et al. A novel mutation (A148V) in the glucose 6-phosphate translocase (SLC37A4) gene in a Korean patient with glycogen storage disease type 1b. J Korean Med Sci. 2005; 20:499–501.
Article16. Chou JY, Matern D, Mansfield BC, Chen YT. Type I glycogen storage diseases: disorders of the glucose-6-phosphatase complex. Curr Mol Med. 2002; 2:121–43.17. Choi R, Park HD, Ko JM, Lee J, Lee DH, Hong SJ, et al. Novel SLC37A4 mutations in Korean patients with glycogen storage disease Ib. Ann Lab Med. 2017; 37:261–6.
Article18. Visser G, Rake JP, Fernandes J, Labrune P, Leonard JV, Moses S, et al. Neutropenia, neutrophil dysfunction, and inflammatory bowel disease in glycogen storage disease type Ib: results of the European Study on Glycogen Storage Disease type I. J Pediatr. 2000; 137:187–91.
Article19. Scheen AJ, Paquot N. Metabolic effects of SGLT-2 inhibitors beyond increased glucosuria: A review of the clinical evidence. Diabetes Metab. 2014; 40:S4–11.
Article