Korean J Lab Med.
2009 Oct;29(5):415-422. 10.3343/kjlm.2009.29.5.415.
Performance Evaluation of Affinity Column Mediated Immunometric Assay for Tacrolimus
- Affiliations
-
- 1Department of Laboratory Medicine, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Korea. sailchun@amc.seoul.kr
- 2Department of Laboratory Medicine, Seoul National University College of Medicine, Seoul, Korea.
- 3Department of Laboratory Medicine, The Catholic University of Korea, College of Medicine, Seoul, Korea.
- KMID: 1077417
- DOI: http://doi.org/10.3343/kjlm.2009.29.5.415
Abstract
- BACKGROUND
Therapeutic drug monitoring (TDM) of tacrolimus is essential because of narrow therapeutic range and poor correlation of dose to blood concentration. Affinity Column Mediated Immunometric Assay (ACMIA) does not require a pretreatment steps in measurement of tacrolimus. In this study, we evaluated the performance of tacrolimus assay using ACMIA (Dimension RxL Max, Dade Behring).
METHODS
The imprecision, the linearity and the detection limits and the interferences by bilirubin and chyle, and correlation with hematocrit for tacrolimus by ACMIA were evaluated according to Clinical and Laboratory Standards Institute guidelines EP5-A2, EP6-A, EP17-A, EP9-A2, and EP7-A2. Method comparison studies with microparticle enzyme immunoassay (MEIA) (IMx Tacrolimus II, Abbott Laboratories) and liquid chromatography-tandem mass spectrometry (LC-MS/MS) (Waters 2795 Quattromicro API, Micromass) were also performed.
RESULTS
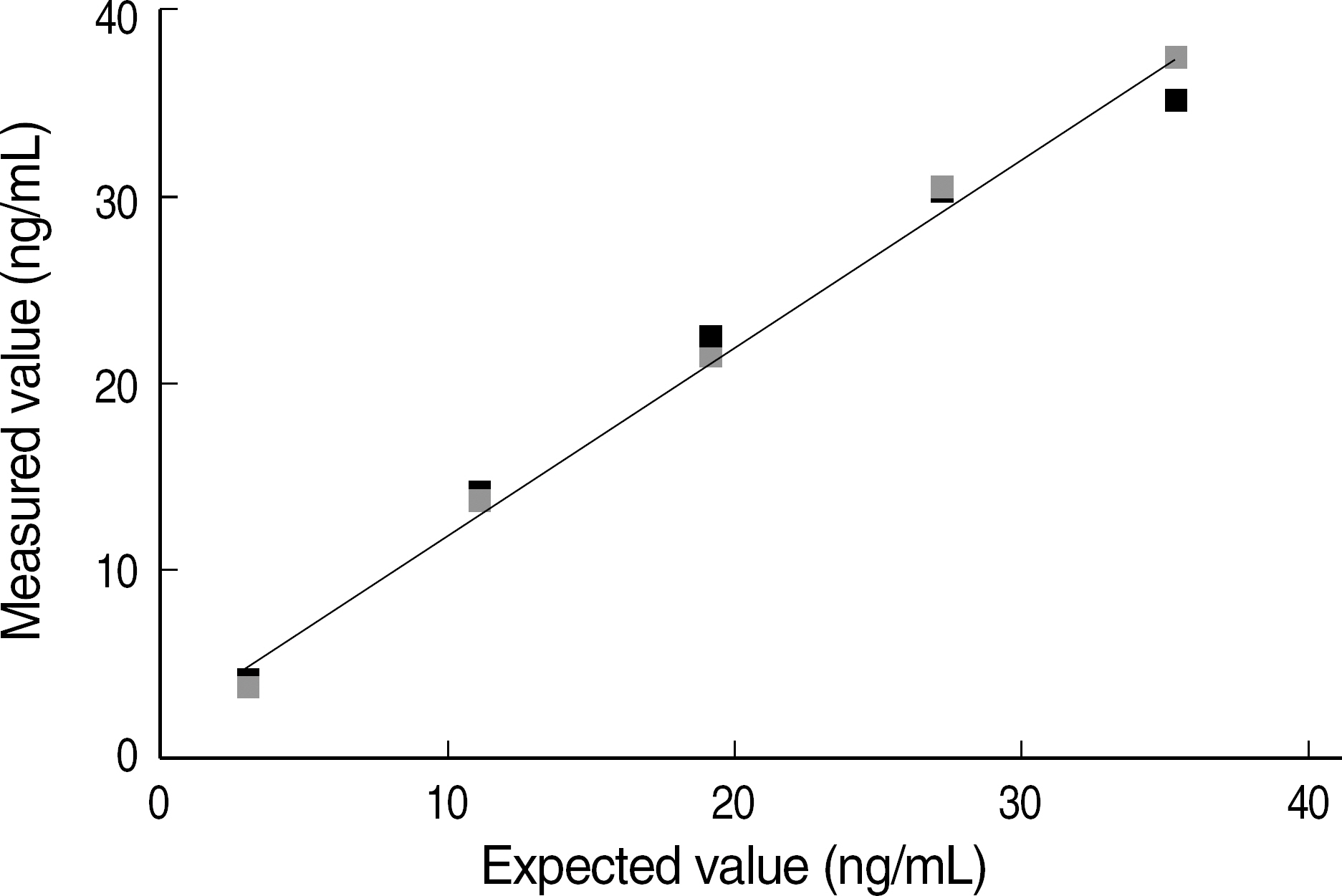
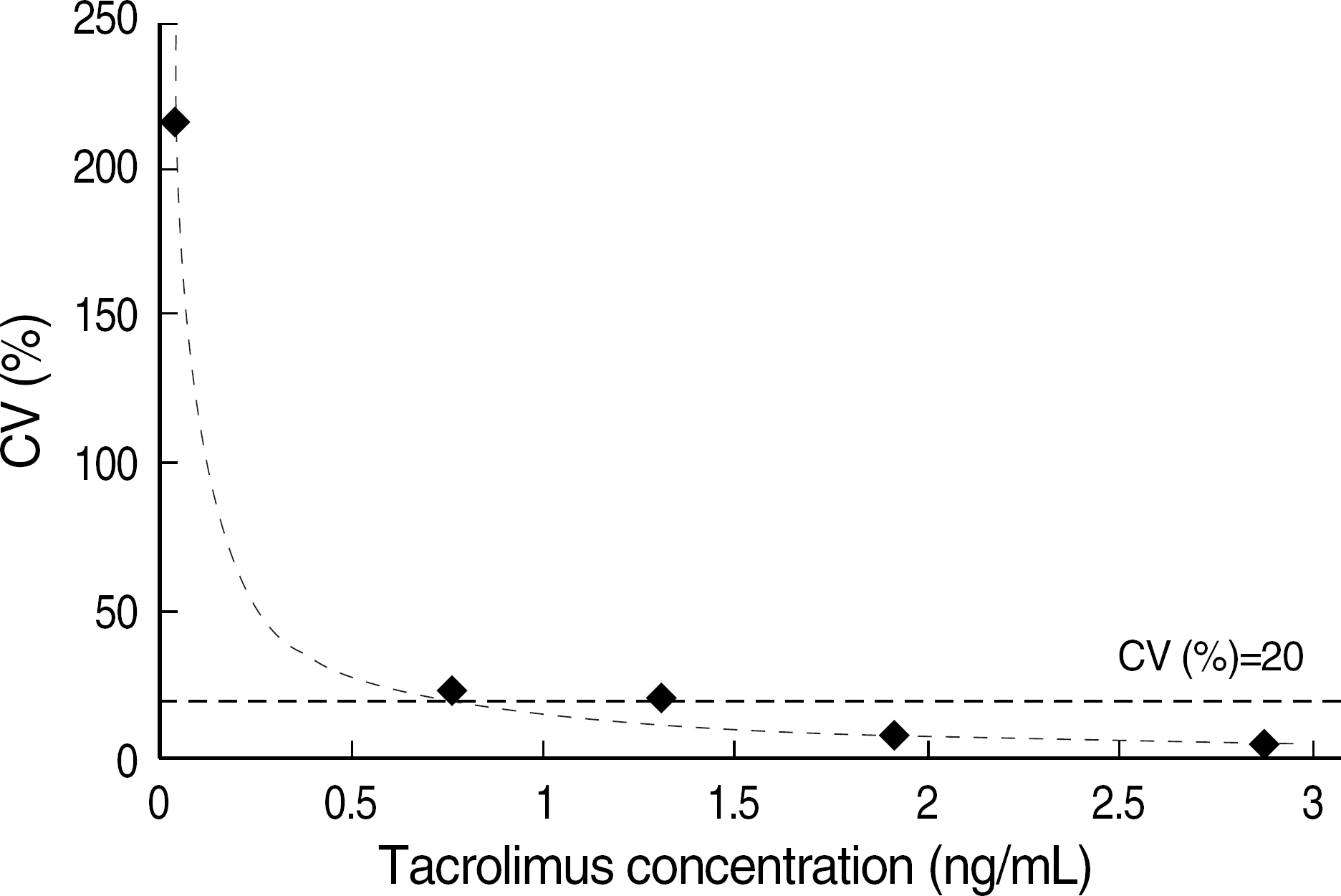
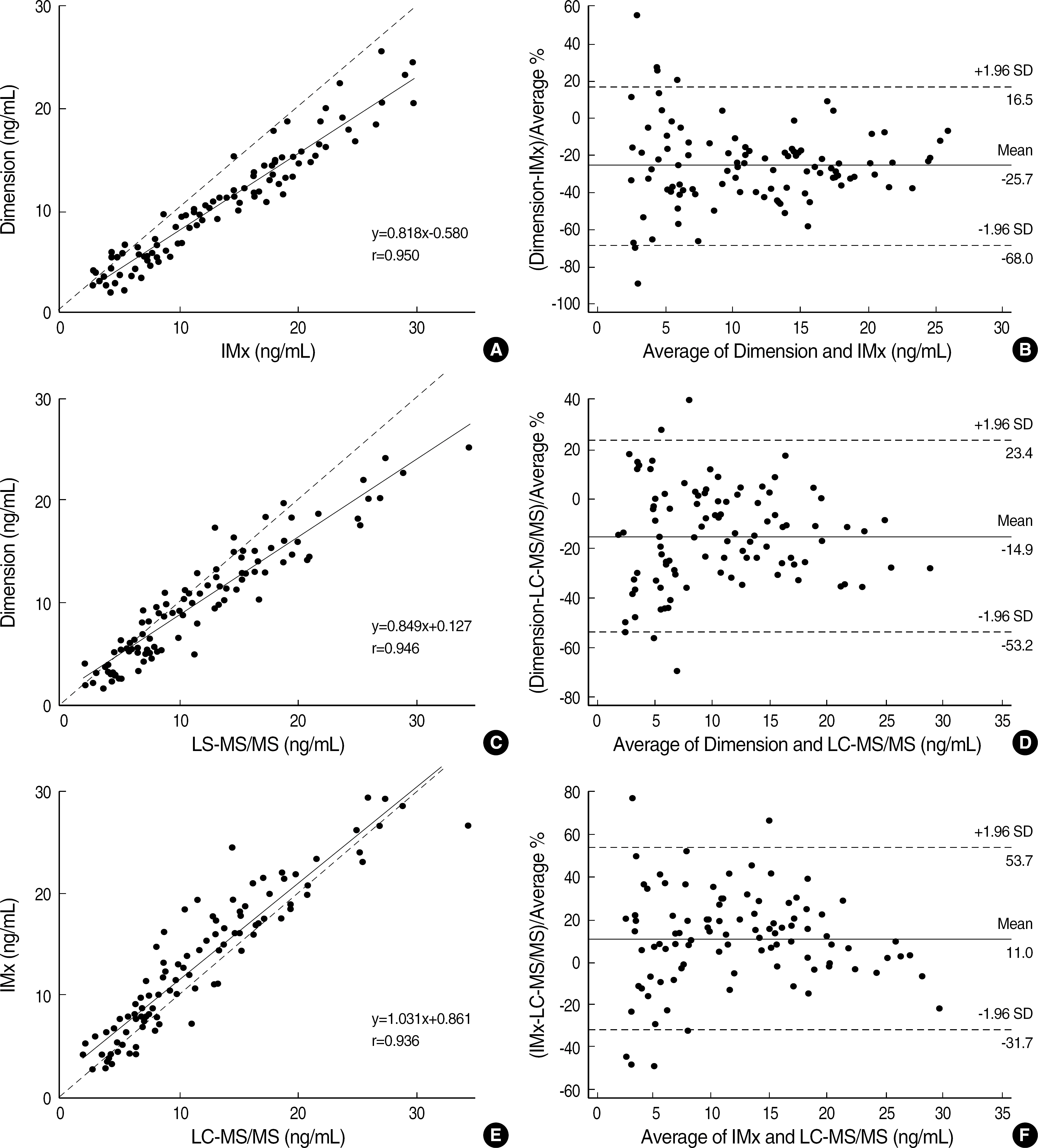
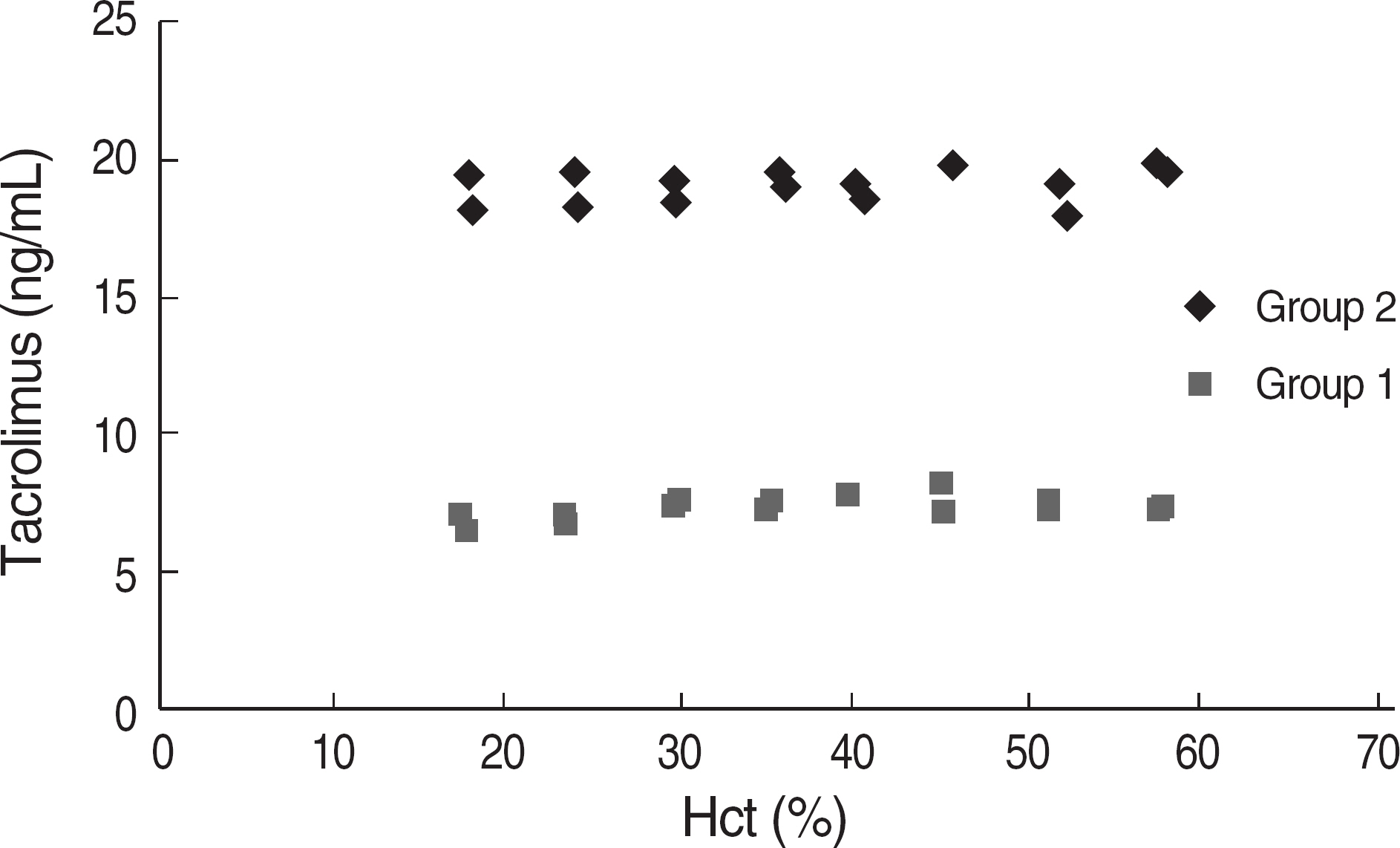
The total imprecision for low, middle and high level was 12.8%, 9.0% and 6.7%, respectively. The range of tacrolimus from 3.1 ng/mL to 35.4 ng/mL showed a clinically relevant linearity. The limit of detection and the functional sensitivity were 0.24 ng/mL and 0.72 ng/mL, respectively. Tacrolimus concentration measurement (Tac-CM) with ACMIA did not show significant interferences with bile and chyle and also did not show significant correlation with hematocrit. In comparison study for Tac-CM with MEIA and LC-MS/MS, Tac-CM with ACMIA showed a good correlation with MEIA (r=0.950) and LC-MS/MS (r=0.946).
CONCLUSIONS
The imprecision, linearity, detection limits, interference and correlation of Tac-CM with ACMIA were suitable for clinical use. Tac-CM with ACMIA could reduce turn around time and help clinicians to manage transplant patients on immunosuppressant therapy.
MeSH Terms
Figure
Cited by 1 articles
-
Falsely Elevated Tacrolimus Concentrations Using Chemiluminescence Microparticle Immunoassay in Kidney Transplant Patient
Dahae Yang, Sae Am Song, Kyung Ran Jun, Hak Rim, Woonhyoung Lee
J Korean Soc Transplant. 2016;30(3):138-142. doi: 10.4285/jkstn.2016.30.3.138.
Reference
-
1.Jusko WJ., Thomson AW., Fung J., McMaster P., Wong SH., Zylber-Katz E, et al. Consensus document: therapeutic monitoring of tacrolimus (FK-506). Ther Drug Monit. 1995. 17:606–14.
Article2.Peters DH., Fitton A., Plosker GL., Faulds D. Tacrolimus. A review of its pharmacology, and therapeutic potential in hepatic and renal transplantation. Drugs. 1993. 46:746–94.3.Nakagawa H., Etoh T., Ishibashi Y., Higaki Y., Kawashima M., Torii H, et al. Tacrolimus ointment for atopic dermatitis. Lancet. 1994. 344:883.
Article4.Westley IS., Taylor PJ., Salm P., Morris RG. Cloned enzyme donor immunoassay tacrolimus assay compared with high-performance liquid chromatography-tandem mass spectrometry and microparticle enzyme immunoassay in liver and renal transplant recipients. Ther Drug Monit. 2007. 29:584–91.
Article5.Ghoshal AK., Soldin SJ. Evaluation of the Dade Behring Dimension RxL: integrated chemistry system-pediatric reference ranges. Clin Chim Acta. 2003. 331:135–46.
Article6.Keown PA. New concepts in cyclosporine monitoring. Curr Opin Nephrol Hypertens. 2002. 11:619–26.
Article7.Kuzuya T., Ogura Y., Motegi Y., Moriyama N., Nabeshima T. Interference of hematocrit in the tacrolimus II microparticle enzyme immunoassay. Ther Drug Monit. 2002. 24:507–11.
Article8.LeGatt DF., Shalapay CE., Cheng SB. The EMIT 2000 tacrolimus assay: an application protocol for the Beckman Synchron LX20 PRO analyzer. Clin Biochem. 2004. 37:1022–30.
Article9.Napoli KL. Is microparticle enzyme-linked immunoassay (MEIA) reliable for use in tacrolimus TDM? Comparison of MEIA to liquid chromatography with mass spectrometric detection using longitudinal trough samples from transplant recipients. Ther Drug Monit. 2006. 28:491–504.
Article10.Taylor PJ., Morris RG. Tacrolimus measurement by microparticle enzyme immunoassay II. Ther Drug Monit. 2003. 25:259–60.
Article11.CSM-B surveys. Immunosuppressive drugs participant summary report, AACC/CAP 2007.12.CSM-A surveys. Immunosuppressive drugs participant summary report, AACC/CAP 2007.13.Kim JH., Kim BK., Lee SY., Chun S., Kwon GC., Yoon Y, et al. Annual report on external quality assessment in therapeutic drug monitoring in Korea (2006). J Lab Med Qual Assur. 2007. 29:121–35. (김정호,김병광, 이수연, 전사일, 권계철, 윤여민 등. TDM 검사 신빙도조사 결과 보고 (2006). 임상검사와정도관리 2007;29:121-35.).14.Clinical and Laboratory Standard Institute. Evaluation of the precision performance of clinical chemistry devices; approved guideline-second edition (EP5-A2). Wayne, PA: Clinical and Laboratory Standards Institute;2004.15.Clinical and Laboratory Standard Institute. Evaluation of the linearity of quantitative measurement procedures: A statistical approach; approved guideline (EP6-A). Wayne, PA: Clinical and Laboratory Standards Institute;2003.16.Kroll MH., Praestgaard J., Michaliszyn E., Styer PE. Evaluation of the extent of nonlinearity in reportable range studies. Arch Pathol Lab Med. 2000. 124:1331–8.
Article17.Clinical and Laboratory Standard Institute. Protocols for determination of limits of detection and limits of quantitation; approved guideline (EP17-A). Wayne, PA: Clinical and Laboratory Standards Institute;2004.18.Westgard JO. Method validation-the detection limit experiment. http://www.westgard.com/lesson29.htm. (Updated on Jun. 2009.19.Clinical and Laboratory Standard Institute. Method comparison and bias estimation using patient samples; approved guideline-second edition (EP9-A2). Wayne, PA: Clinical and Laboratory Standards Institute;2002.20.Clinical and Laboratory Standard Institute. Interference testing in clinical chemistry; approved guideline-second edition (EP7-A2). Wayne, PA: Clinical and Laboratory Standards Institute;2005.21.Guidance for Industry Bioanalytical Method Validation. U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center for Veterinary Medicine (CVM). http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM070107.pdf. (accessed September. 2009.22.Dorizzi RM., Cocco C., Rizzotti P. Tacrolimus assays; new tools for new tests and for old problems. Clin Chim Acta. 2008. 387:177–8.
Article23.Griffey MA., Hock KG., Kilgore DC., Wei TQ., Duh SH., Christenson R, et al. Performance of a no-pretreatment tacrolimus assay on the Dade Behring Dimension RxL clinical chemistry analyzer. Clin Chim Acta. 2007. 384:48–51.
Article24.Cogill JL., Taylor PJ., Westley IS., Morris RG., Lynch SV., Johnson AG. Evaluation of the tacrolimus II microparticle enzyme immunoassay (MEIA II) in liver and renal transplant recipients. Clin Chem. 1998. 44:1942–6.
Article25.Iwasaki K., Shiraga T., Matsuda H., Nagase K., Tokuma Y., Hata T, et al. Further metabolism of FK506 (tacrolimus). Identification and biological activities of the metabolites oxidized at multiple sites of FK506. Drug Metab Dispos. 1995. 23:28–34.26.Ghoshal AK., Soldin SJ. IMx tacrolimus II assay: is it reliable at low blood concentrations? A comparison with tandem MS/MS. Clin Biochem. 2002. 35:389–92.
Article27.Tomita T., Homma M., Yuzawa K., Ohkohchi N., Hori T., Kaneko M, et al. Effects of hematocrit value on microparticle enzyme immunoassay of tacrolimus concentration in therapeutic drug monitoring. Ther Drug Monit. 2005. 27:94–7.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Performance Evaluation of MassTrak LC/MS/MS Tacrolimus Kit
- Purification of Opioid Receptor in the Presence of Sodium Ion
- Purification of human RBC insulin receptor by high performance insulin affinity column
- Performance Evaluation of the ARCHITECT i2000 for the Determination of Whole Blood Cyclosporin A and Tacrolimus
- Two Cases of Lichen Nitidus Treated with Topical 0.1% Tacrolimus