J Vet Sci.
2010 Dec;11(4):327-332. 10.4142/jvs.2010.11.4.327.
Optimization of culture media of pathogenic Mycoplasma hyopneumoniae by a response surface methodology
- Affiliations
-
- 1College of Veterinary Medicine, Kyungpook National University, Daegu 702-701, Korea. parksch@knu.ac.kr
- 2Gyeongbuk Veterinary Service Laboratory, Daegu 702-210, Korea.
- KMID: 1072182
- DOI: http://doi.org/10.4142/jvs.2010.11.4.327
Abstract
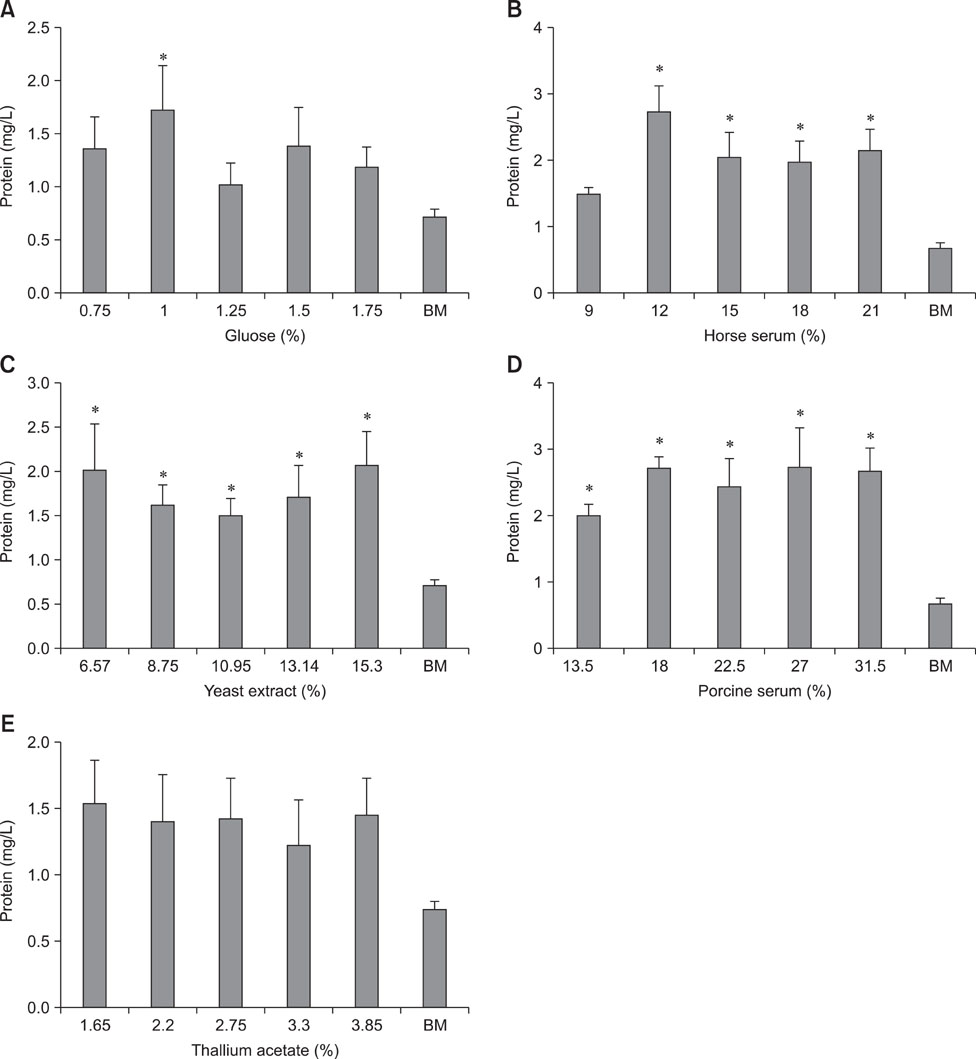
- Composition of culture medium for mass production of Mycoplasma hyopneumoniae was optimized using a response surface methodology (RSM). Initially, the influence of glucose, thallium acetate, fresh yeast extract, horse serum, and porcine serum on the production of mycoplasmal protein was assessed using a 'one factor at a time' technique. Next, factors with a significant effect, including fresh yeast extract, and horse and porcine sera, were selected for further optimization using a central composite design (CCD) of RSM. The experimental results were fitted into a second order polynomial model equation. Estimated optimal condition of the factors for maximum production of mycoplasmal protein (i.e., triple-fold increase from 0.8 mg/L produced by basal mycoplasma media to 2.5 mg/L) was 10.9% fresh yeast extract, 15% horse serum, and 31.5% porcine serum (v/v). For the optimized conditions, a 2.96 mg/L experimental result was observed, similar to the estimated optimal conditions result of the CCD.
Keyword
MeSH Terms
Figure
Reference
-
1. Amass SF, Clark LK, Van Alstine WG, Bowersock TL, Murphy DA, Knox KE, Albregts SR. Interaction of Mycoplasma hyopneumoniae and Pasteurella multocida infections in swine. J Am Vet Med Assoc. 1994. 204:102–107.2. Angulo AF, Jacobs MV, van Damme EH, Akkermans AM, de Kruijff-Kroesen I, Brugman J. Colistin sulfate as a suitable substitute of thallium acetate in culture media intended for mycoplasma detection and culture. Biologicals. 2003. 31:161–163.
Article3. DeBey MC, Jacobson CD, Ross RF. Histochemical and morphologic changes of porcine airway epithelial cells in response to infection with Mycoplasma hyopneumoniae. Am J Vet Res. 1992. 53:1705–1710.4. Duta FP, De França FP, Sérvulo EF, De Almeida Lopes LM, Da Costa AC, Barros A. Effect of process parameters on production of a biopolymer by Rhizobium sp. Appl Biochem Biotechnol. 2004. 113-116:639–652.5. Fischer O. Enhancement of propagation of Mycoplasma hyopneumoniae by culture in a biphasic medium. Acta Vet Brno. 1995. 64:243–247.
Article6. Friis NF. Some recommendations concerning primary isolation of Mycoplasma suipneumoniae and Mycoplasma flocculare a survey. Nord Vet Med. 1975. 27:337–339.7. Friis NF, Szancer J. Sensitivity of certain porcine and bovine mycoplasmas to antimicrobial agents in a liquid medium test compared to a disc assay. Acta Vet Scand. 1994. 35:389–394.
Article8. Garner CM, Hubbold LM, Chakraborti PR. Mycoplasma detection in cell cultures: a comparison of four methods. Br J Biomed Sci. 2000. 57:295–301.9. Hwang MH, Lim JH, Yun HI, Kim JC, Jung BY, Hsu WH, Park SC. The effect of polyclonal antibody on intracellular calcium increase induced by Mycoplasma hyopneumoniae in porcine tracheal cells. Vet J. 2006. 172:556–560.
Article10. Li C, Bai J, Cai Z, Ouyang F. Optimization of a cultural medium for bacteriocin production by Lactococcus lactis using response surface methodology. J Biotechnol. 2002. 93:27–34.
Article11. Lim YT, Seok HB. Studies on the mycoplasmal pneumonia in slaughter pigs. 1. Seasonal detection by gross finding of lung lesion and dot-ELISA technique. Korean J Vet Res. 2002. 42:219–224.12. McGowin CL, Ma L, Martin DH, Pyles RB. Mycoplasma genitalium-encoded MG309 activates NF-κB via Toll-like receptors 2 and 6 to elicit proinflammatory cytokine secretion from human genital epithelial cells. Infect Immun. 2009. 77:1175–1181.
Article13. Mu W, Chen C, Li X, Zhang T, Jiang B. Optimization of culture medium for the production of phenyllactic acid by Lactobacillus sp. SK007. Bioresour Technol. 2009. 100:1366–1370.
Article14. Park SC, Yibchok-Anun S, Cheng H, Young TF, Thacker EL, Minion FC, Ross RF, Hsu WH. Mycoplasma hyopneumoniae increases intracellular calcium release in porcine ciliated tracheal cells. Infect Immun. 2002. 70:2502–2506.
Article15. Rogers MJ, Simmons J, Walker RT, Weisburg WG, Woese CR, Tanner RS, Robinson IM, Stahl DA, Olsen G, Leach RH, Maniloff J. Construction of the mycoplasma evolutionary tree from 5s rRNA sequence data. Proc Natl Acad Sci USA. 1985. 82:1160–1164.
Article16. Sunitha K, Chung BH, Jang KH, Song KB, Kim CH, Rhee SK. Refolding and purification of Zymomonas mobilis levansucrase produced as inclusion bodies in fed-batch culture of recombinant Escherichia coli. Protein Expr Purif. 2000. 18:388–393.
Article17. Thacker EL. Diagnosis of Mycoplasma hyopneumoniae. Anim Health Res Rev. 2004. 5:317–320.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- T helper 1-type immunogenicity of Mycoplasma hyopneumoniae antigen on mouse spleen cells
- A sensitive and specific polymerase chain reaction to detect Mycoplasma hyopnemoniae using Mycoplasma protein P97 gene
- An improved multiplex PCR for diagnosis and differentiation of Mycoplasma hyopneumoniae and Mycoplasma hyorhinis
- In vitro antibiotic susceptibility of field isolates of Mycoplasma hyopneumoniae and Mycoplasma hyorhinis from Korea
- Bordetella bronchiseptica antigen enhances the production of Mycoplasma hyopneumoniae antigen-specific immunoglobulin G in mice