J Vet Sci.
2010 Dec;11(4):315-319. 10.4142/jvs.2010.11.4.315.
Functional activities of the Tsh protein from avian pathogenic Escherichia coli (APEC) strains
- Affiliations
-
- 1Departamento de Microbiologia-CCB, Campus Universitario, Universidade Estadual de Londrina, Caixa postal 6001, 86051-970 Londrina, Parana, Brazil.
- 2Departamento de Medicina Veterinaria Preventiva-CCA, Campus Universitario, Universidade Estadual de Londrina, Caixa postal 6001, 86051-970 Londrina, Parana, Brazil. kobayashirkt@uel.br
- KMID: 1072180
- DOI: http://doi.org/10.4142/jvs.2010.11.4.315
Abstract
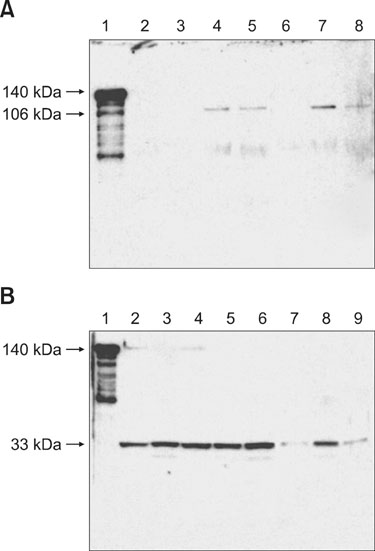
- The temperature-sensitive hemagglutinin (Tsh) expressed by strains of avian pathogenic Escherichia (E.) coli (APEC) has both agglutinin and protease activities. Tsh is synthesized as a 140 kDa precursor protein, whose processing results in a 106 kDa passenger domain (Tsh(s)) and a 33 kDa beta-domain (Tsh(beta)). In this study, both recombinant Tsh (rTsh) and supernatants from APEC, which contain Tsh(s) (106 kDa), caused proteolysis of chicken tracheal mucin. Both rTsh (140 kDa) and pellets from wild-type APEC, which contain Tsh(beta) (33 kDa), agglutinated chicken erythrocytes. On Western blots, the anti-rTsh antibody recognized the rTsh and 106 kDa proteins in recombinant E. coli BL21/pET 101-Tsh and in the supernatants from APEC grown at either 37degrees C or 42degrees C. Anti-rTsh also recognized a 33 kDa protein in the pellets from APEC13 cultures grown in either Luria-Bertani agar, colonization factor antigen agar, or mucin agar at either 26degrees C, 37degrees C, or 42degrees C, and in the extracts of outer membrane proteins of APEC. The 106 kDa protein was more evident when the bacteria were grown at 37degrees C in mucin agar, and it was not detected when the bacteria were grown at 26degrees C in any of the culture media used in this study. Chicken anti-Tsh serum inhibited hemagglutinating and mucinolytic activities of strain APEC13 and recombinant E. coli BL21/pET101-Tsh. This work suggests that the mucinolytic activity of Tsh might be important for the colonization of the avian tracheal mucous environment by APEC.
Keyword
MeSH Terms
Figure
Reference
-
1. Benitez JA, Silva AJ, Finkelstein RA. Environmental signals controlling production of hemagglutinin/protease in Vibrio cholerae. Infect Immun. 2001. 69:6549–6553.
Article2. de Moura AC, Irino K, Vidotto MC. Genetic variability of avian Escherichia coli strains evaluated by enterobacterial repetitive intergenic consensus and repetitive extragenic palindromic polymerase chain reaction. Avian Dis. 2001. 45:173–181.
Article3. Delicato ER, de Brito BG, Konopatzki AP, Gaziri LC, Vidotto MC. Occurrence of the temperature-sensitive hemagglutinin among avian Escherichia coli. Avian Dis. 2002. 46:713–716.
Article4. Dozois CM, Dho-Moulin M, Brée A, Fairbrother JM, Desautels C, Curtiss R III. Relationship between the Tsh autotransporter and pathogenicity of avian Escherichia coli and localization and analysis of the Tsh genetic region. Infect Immun. 2000. 68:4145–4154.
Article5. Dutta PR, Cappello R, Navarro-García F, Nataro JP. Functional comparison of serine protease autotransporters of Enterobacteriaceae. Infect Immun. 2002. 70:7105–7113.
Article6. Griffiths E, Stevenson P, Joyce P. Pathogenic Escherichia coli express new outer membrane proteins when growing in vivo. FEMS Microbiol Lett. 1983. 16:95–99.
Article7. Henderson IR, Navarro-Garcia F, Nataro JP. The great escape: structure and function of the autotransporter proteins. Trends Microbiol. 1998. 6:370–378.
Article8. Kobayashi RKT, Gaziri LCJ, Venancio EJ, Vidotto MC. Detection of Tsh protein mucinolytic activity by SDS-PAGE. J Microbiol Methods. 2007. 68:654–655.
Article9. Kostakioti M, Stathopoulos C. Functional analysis of the Tsh autotransporter from an avian pathogenic Escherichia coli strain. Infect Immun. 2004. 72:5548–5554.
Article10. Maurer JJ, Brown TP, Steffens WL, Thayer SG. The occurrence of ambient temperature-regulated adhesins, curli, and the temperature-sensitive hemagglutinin Tsh among avian Escherichia coli. Avian Dis. 1998. 42:106–118.
Article11. Miller GL. Protein determination for large numbers of samples. Analyt Chem. 1959. 31:964.12. Provence DL, Curtiss R III. Role of crl in avian pathogenic Escherichia coli: a knockout mutation of crl does not affect hemagglutination activity, fibronectin binding, or curli production. Infect Immun. 1992. 60:4460–4467.
Article13. Provence DL, Curtiss R III. Isolation and characterization of a gene involved in hemagglutination by an avian pathogenic Escherichia coli strain. Infect Immun. 1994. 62:1369–1380.
Article14. Simões RC, Kobayashi RKT, Gaziri LCJ, Vidotto MC. Cloning, sequencing, expression, and characterization of the Tsh gene from an avian pathogenic Escherichia coli strain. Semina: Ciências Agrárias. 2006. 27:253–260.
Article15. Stathopoulos C, Provence DL, Curtiss R III. Characterization of the avian pathogenic Escherichia coli hemagglutinin Tsh, a member of the immunoglobulin A protease-type family of autotransporters. Infect Immun. 1999. 67:772–781.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Epidemiological Prevalence of Avian Pathogenic Escherichia coli Differentiated by Multiplex PCR from Commercial Chickens and Hatchery in Korea
- Pathotyping avian pathogenic Escherichia coli strains in Korea
- rpoB gene sequencing for phylogenetic analysis of avian pathogenic Escherichia coli
- Hens immunized with live attenuated Salmonella strains expressing virulence-associated genes in avian pathogenic Escherichia coli passively transfer maternal antibodies to chicks
- Evaluation of systemic and mucosal immune responses in mice administered with novel recombinant Salmonella vaccines for avian pathogenic Esherichia coli