J Vet Sci.
2011 Mar;12(1):21-25. 10.4142/jvs.2011.12.1.21.
Determination of angiotensin I-converting enzyme activity in equine blood: lack of agreement between methods of analysis
- Affiliations
-
- 1Faculty of Veterinary Science, The University of Melbourne, Victoria 3010, Australia. Fernanda.Costa@wintec.ac.nz
- 2BIO 21 Institute, The University of Melbourne, Victoria 3010, Australia.
- 3Universidade Federal de Sao Paulo, Sao Paulo 04023-900, Brazil.
- KMID: 1067333
- DOI: http://doi.org/10.4142/jvs.2011.12.1.21
Abstract
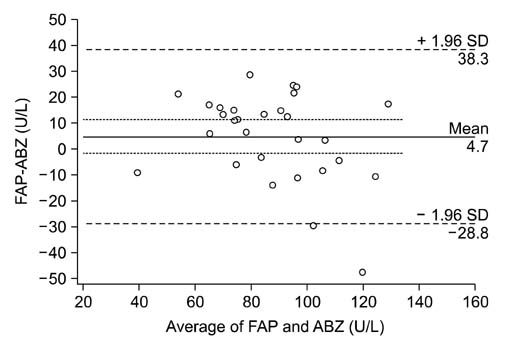
- Angiotensin-I converting enzyme (ACE) is a key regulator of blood pressure, electrolytes and fluid homeostasis through conversion of angiotensin I into angiotensin II. Recently, a genetic polymorphism of the ACE gene, which accounts for 47% of the variation of ACE activity in blood, has been advocated as a biomarker of athletic aptitude. Different methods of analysis and determination of ACE activity in plasma have been used in human and equine research without a consensus of a "gold standard" method. Different methods have often been used interchangeably or cited as being comparable in the existing literature; however, the actual agreement between assays has not been investigated. Therefore, in this study, we evaluated the level of agreement between three different assays using equine plasma obtained from 29 horses. Two spectrophotometric assays using Furylacryloyl-phenylalanyl-glycyl-glycine as substrate and one fluorimetric assay utilizing o-aminobenzoic acid-FRK-(Dnp)P-OH were employed. The results revealed that the measurements from the different assays were not in agreement, indicating that the methods should not be used interchangeably for measurement of equine ACE activity. Rather, a single method of analysis should be adopted to achieve comparable results and critical appraisal of the literature is needed when attempting to compare results obtained from different assays.
MeSH Terms
Figure
Reference
-
1. Alves MF, Araujo MC, Juliano MA, Oliveira EM, Krieger JE, Casarini DE, Juliano L, Carmona AK. A continuous fluorescent assay for the determination of plasma and tissue angiotensin I-converting enzyme activity. Braz J Med Biol Res. 2005. 38:861–868.
Article2. Araujo MC, Melo RL, Cesari MH, Juliano MA, Juliano L, Carmona AK. Peptidase specificity characterization of C- and N-terminal catalytic sites of angiotensin I-converting enzyme. Biochemistry. 2000. 39:8519–8525.
Article3. Ball BA, Gravance CG, Wessel MT, Sabeur K. Activity of angiotensin-converting enzyme (ACE) in reproductive tissues of the stallion and effects of angiotensin II on sperm motility. Theriogenology. 2003. 59:901–914.
Article4. BENCH (BENazepril in Canine Heart disease) Study Group. The effect of benazepril on survival times and clinical signs of dogs with congestive heart failure: Results of a multicenter, prospective, randomized, double-blinded, placebo-controlled, long-term clinical trial. J Vet Cardiol. 1999. 1:7–18.5. Beneteau B, Baudin B, Morgant G, Giboudeau J, Baumann FC. Automated kinetic assay of angiotensin-converting enzyme in serum. Clin Chem. 1986. 32:884–886.
Article6. Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999. 8:135–160.
Article7. Bramucci M, Miano A, Quassinti L, Maccari E, Murri O, Amici D. Presence and comparison of Angiotensin converting enzyme in commercial cell culture sera. Biochem Mol Biol Int. 1999. 47:107–115.
Article8. Carmona AK, Schwager SL, Juliano MA, Juliano L, Sturrock ED. A continuous fluorescence resonance energy transfer angiotensin I-converting enzyme assay. Nat Protoc. 2006. 1:1971–1976.
Article9. Coomer RPC, Forhead AJ, Bathe AP, Head MJ. Plasma angiotensin-converting enzyme (ACE) concentration in Thoroughbred racehorses. Equine Vet J. 2003. 35:96–98.
Article10. Costa MFM. Angiotensin-converting enzyme (ACE) in horses and its relationship to performance and fitness. Ph.D dissertation. 2010. Melbourne: The University of Melbourne.11. Cushman DW, Cheung HS. Spectrophotometric assay and properties of the angiotensin-converting enzyme of rabbit lung. Biochem Pharmacol. 1971. 20:1637–1648.
Article12. Ellis N. Molecular genetic characterization of the equine angiotensin-converting enzyme gene. Faculty of Veterinary Science. 2005. Sydney: The University of Sydney;338.13. Fogarty Y, Fraser CG, Browning MCK. Intra- and inter-individual variation of serum angiotensin converting enzyme: clinical implications. Ann Clin Biochem. 1989. 26:201–202.
Article14. Forhead AJ, Gillespie CE, Fowden AL. Role of cortisol in the ontogenic control of pulmonary and renal angiotensin-converting enzyme in fetal sheep near term. J Physiol. 2000. 526:409–416.
Article15. Ganjam VK, Evans TJ. Equine endometrial fibrosis correlates with 11β-HSD2, TGF-β1 and ACE activities. Mol Cell Endocrinol. 2006. 248:104–108.
Article16. Gardner SY, Atkins CE, Sams RA, Schwabenton AB, Papich MG. Characterization of the pharmacokinetic and pharmacodynamic properties of the angiotensin-converting enzyme inhibitor, enalapril, in horses. J Vet Intern Med. 2004. 18:231–237.
Article17. Hurst PL, Lovell-Smith CJ. Optimized assay for serum angiotensin-converting enzyme activity. Clin Chem. 1981. 27:2048–2052.
Article18. Mantha S, Roizen MF, Fleisher LA, Thisted R, Foss J. Comparing methods of clinical measurement: reporting standards for Bland and Altman analysis. Anesth Analg. 2000. 90:593–602.
Article19. Montgomery H, Clarkson P, Barnard M, Bell J, Brynes A, Dollery C, Hajnal J, Hemingway H, Mercer D, Jarman P, Marshall R, Prasad K, Rayson M, Saeed N, Talmud P, Thomas L, Jubb M, World M, Humphries S. Angiotensin-converting-enzyme gene insertion/deletion polymorphism and response to physical training. Lancet. 1999. 353:541–545.
Article20. Muir WW III, Sams RA, Hubbell JAE, Hinchcliff KW, Gadawski J. Effects of enalaprilat on cardiorespiratory, hemodynamic, and hematologic variables in exercising horses. Am J Vet Res. 2001. 62:1008–1013.
Article21. Myerson S, Hemingway H, Budget R, Martin J, Humphries S, Montgomery H. Human angiotensin I-converting enzyme gene and endurance performance. J Appl Physiol. 1999. 87:1313–1316.22. Nocenti MR, Simchon S, Cizek LJ. Analysis of the renin-angiotensin system during fasting in adult male rabbits. Proc Soc Exp Biol Med. 1975. 150:142–147.
Article23. O'Connor SJ, Fowden AL, Holdstock N, Giussani DA, Forhead AJ. Developmental changes in pulmonary and renal angiotensin-converting enzyme concentration in fetal and neonatal horses. Reprod Fertil Dev. 2002. 14:413–417.24. Raimbach SJ, Thomas AL. Renin and angiotensin converting enzyme concentrations in the fetal and neonatal guinea-pig. J Physiol. 1990. 423:441–451.
Article25. Rigat B, Hubert C, Alhenc-Gelas F, Cambien F, Corvol P, Soubrier F. An insertion/deletion polymorphism in the angiotensin I-converting enzyme gene accounting for half the variance of serum enzyme levels. J Clin Invest. 1990. 86:1343–1346.
Article26. Ronca-Testoni S. Direct spectrophotometric assay for Angiotensin-converting enzyme in serum. Clin Chem. 1983. 29:1093–1096.
Article27. Sabatini RA, Bersanetti PA, Farias SL, Juliano L, Juliano MA, Casarini DE, Carmona AK, Paiva ACM, Pesquero JB. Determination of angiotensin I-converting enzyme activity in cell culture using fluorescence resonance energy transfer peptides. Anal Biochem. 2007. 363:255–262.
Article28. Schweisfurth H, Schiöberg-Schiegnitz S. Assay and biochemical characterization of angiotensin-I-converting enzyme in cerebrospinal fluid. Enzyme. 1984. 32:12–19.
Article29. Tillman LG, Moore JN. Serum angiotensin converting enzyme activity and response to angiotensin I in horses. Equine Vet J. 1989. 21:Suppl 7. s80–s83.
Article30. Tiret L, Rigat B, Visvikis S, Breda C, Corvol P, Cambien F, Soubrier F. Evidence, from combined segregation and linkage analysis, that a variant of the angiotensin I-converting enzyme (ACE) gene controls plasma ACE levels. Am J Hum Genet. 1992. 51:197–205.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Angiotensin Converting Enzyme Inhibitors on Induced Angiotensin Converting Enzyme Activity in Rat Intestine
- Angiotensin Converting Enzyme Inhibitors for the
- Overview of the Renin-Angiotensin System
- ACE Inhibitors and Losartan in the Managerment of Hypertesion
- Angiotensin converting enzyme inhibitors remain the first treatment of choice