Korean J Radiol.
2012 Feb;13(1):111-114. 10.3348/kjr.2012.13.1.111.
Giant High-Flow Type Pulmonary Arteriovenous Malformation: Coil Embolization with Flow Control by Balloon Occlusion and an Anchored Detachable Coil
- Affiliations
-
- 1Department of Radiology, Gifu University Hospital, Gifu 501-1194, Japan. masa_gif@yahoo.co.jp
- 2Research Center for Cancer Prevention and Screening, National Cancer Center Hospital, Tokyo 104-0045, Japan.
- KMID: 1058803
- DOI: http://doi.org/10.3348/kjr.2012.13.1.111
Abstract
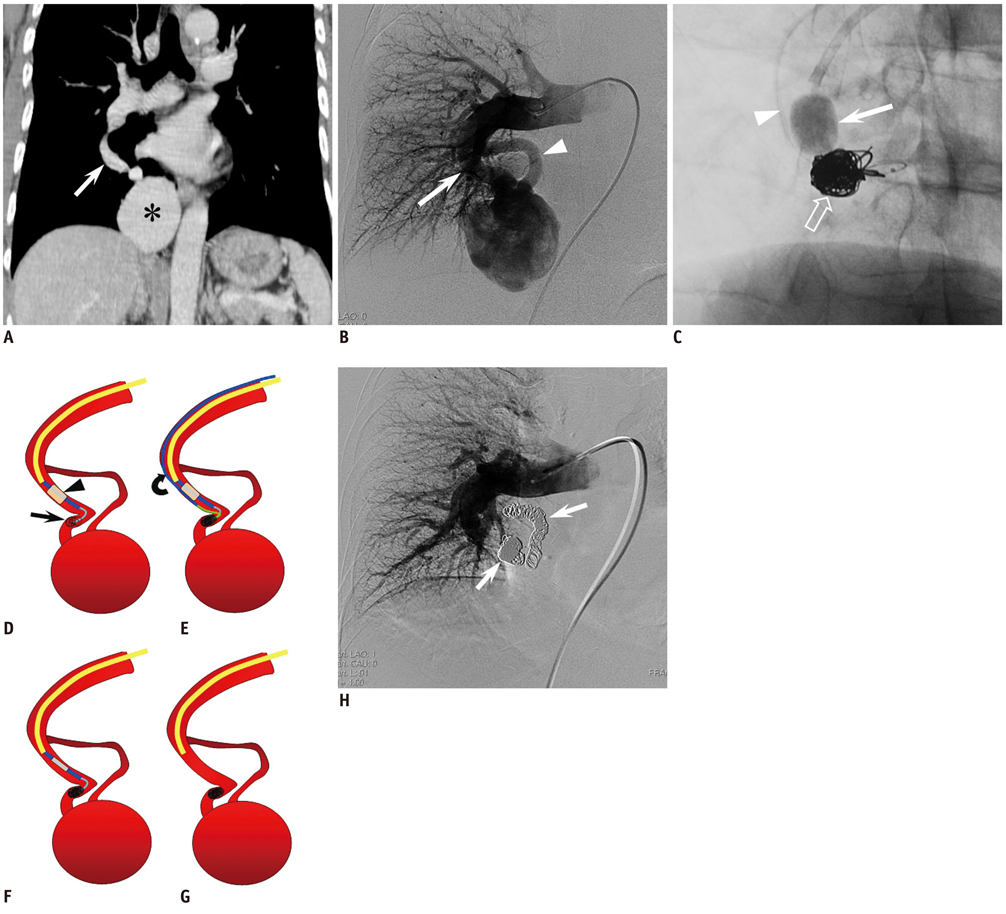
- Pulmonary arteriovenous malformations (PAVMs) are often treated by pushable fibered or non-fibered microcoils, using an anchor or scaffold technique or with an Amplatzer plug through a guiding sheath. When performing percutaneous transcatheter microcoil embolization, there is a risk of coil migration, particularly with high-flow type PAVMs. The authors report on a unique treatment in a patient with a giant high-flow PAVM whose nidus had a maximum diameter of 6 cm. A detachable coil, not detached from a delivery wire (an anchored detachable coil), was first placed in the feeding artery under flow control by balloon occlusion, and then multiple microcoils were packed proximally to the anchored detachable coil. After confirming the stability of the microcoils during a gradual deflation of the balloon, we finally released the first detachable coil. The nidus was reduced in size to 15 mm at one year postoperatively.
Keyword
MeSH Terms
Figure
Reference
-
1. Sluiter-Eringa H, Orie NG, Sluiter HJ. Pulmonary arteriovenous fistula. Diagnosis and prognosis in noncomplainant patients. Am Rev Respir Dis. 1969. 100:177–188.2. Fellows KE. What is an arteriovenous malformation? Cardiovasc Intervent Radiol. 1987. 10:53–54.3. White RI Jr, Lynch-Nyhan A, Terry P, Buescher PC, Farmlett EJ, Charnas L, et al. Pulmonary arteriovenous malformations: techniques and long-term outcome of embolotherapy. Radiology. 1988. 169:663–669.4. Remy J, Remy-Jardin M, Wattinne L, Deffontaines C. Pulmonary arteriovenous malformations: evaluation with CT of the chest before and after treatment. Radiology. 1992. 182:809–816.5. Ference BA, Shannon TM, White RI Jr, Zawin M, Burdge CM. Life-threatening pulmonary hemorrhage with pulmonary arteriovenous malformations and hereditary hemorrhagic telangiectasia. Chest. 1994. 106:1387–1390.6. Dutton JA, Jackson JE, Hughes JM, Whyte MK, Peters AM, Ussov W, et al. Pulmonary arteriovenous malformations: results of treatment with coil embolization in 53 patients. AJR Am J Roentgenol. 1995. 165:1119–1125.7. Ethical principles for medical research involving human subjects. World medical association declaration of Helsinki. Accessed on August 1, 2011. http://ohsr.od.nih.gov/guidelines/helsinki.html.8. Moussouttas M, Fayad P, Rosenblatt M, Hashimoto M, Pollak J, Henderson K, et al. Pulmonary arteriovenous malformations: cerebral ischemia and neurologic manifestations. Neurology. 2000. 55:959–964.9. Mori K, Shiigai M, Saida T, Anno I, Wada M, Minami M. A modified metallic coil embolization technique for pulmonary arteriovenous malformations using coil anchors and occlusion balloon catheters. Cardiovasc Intervent Radiol. 2008. 31:638–642.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Coil Embolization of High-flow Pial Arteriovenous Fistula and Management of Hyperperfusion Syndrome: a Case Report
- A Case of Multiple Pulmonary Arteriovenous Malformation Treated with Coil Embolization
- GDC(Guglielmi Detachable Coil) Embolization Used in Carotid-Cavernous Fistula Incompletely Occluded by Detachable Balloon
- A Case of Giant Aneurysm of Coronary Arteriovenous Fistula Treated by Percutaneous Deployment of Embolization Coil
- A Case of Embolotherapy of Diffuse Pulmonary Arteriovenous Malformation Using Amplatzer Vascular Plugs