Blood Res.
2020 Dec;55(4):206-212. 10.5045/br.2020.2020158.
Safety and efficacy analysis of ibrutinib in 32 patients with CLL and various B-cell lymphomas: real-world data from a single-center study in Turkey
- Affiliations
-
- 1Department of Hematology, Antalya Training and Research Hospital, Antalya, Turkey
- KMID: 2509959
- DOI: http://doi.org/10.5045/br.2020.2020158
Abstract
- Background
Ibrutinib is an oral irreversible Bruton’s tyrosine kinase inhibitor. Here, we demonstrate the efficacy and safety of ibrutinib using real-life data from patients with marginal zone lymphoma (MZL), diffuse large B-cell lymphoma (DLBCL), Waldenström macroglobulinemia (WM), and follicular lymphoma (FL), especially in chronic lymphocytic leukemia (CLL) and mantle cell lymphoma (MCL).
Methods
This is a retrospective, observational, non-interventional, and single-center study on 32 patients who received ibrutinib treatment between January 2017 and March 2020 regardless of their diagnosis.
Results
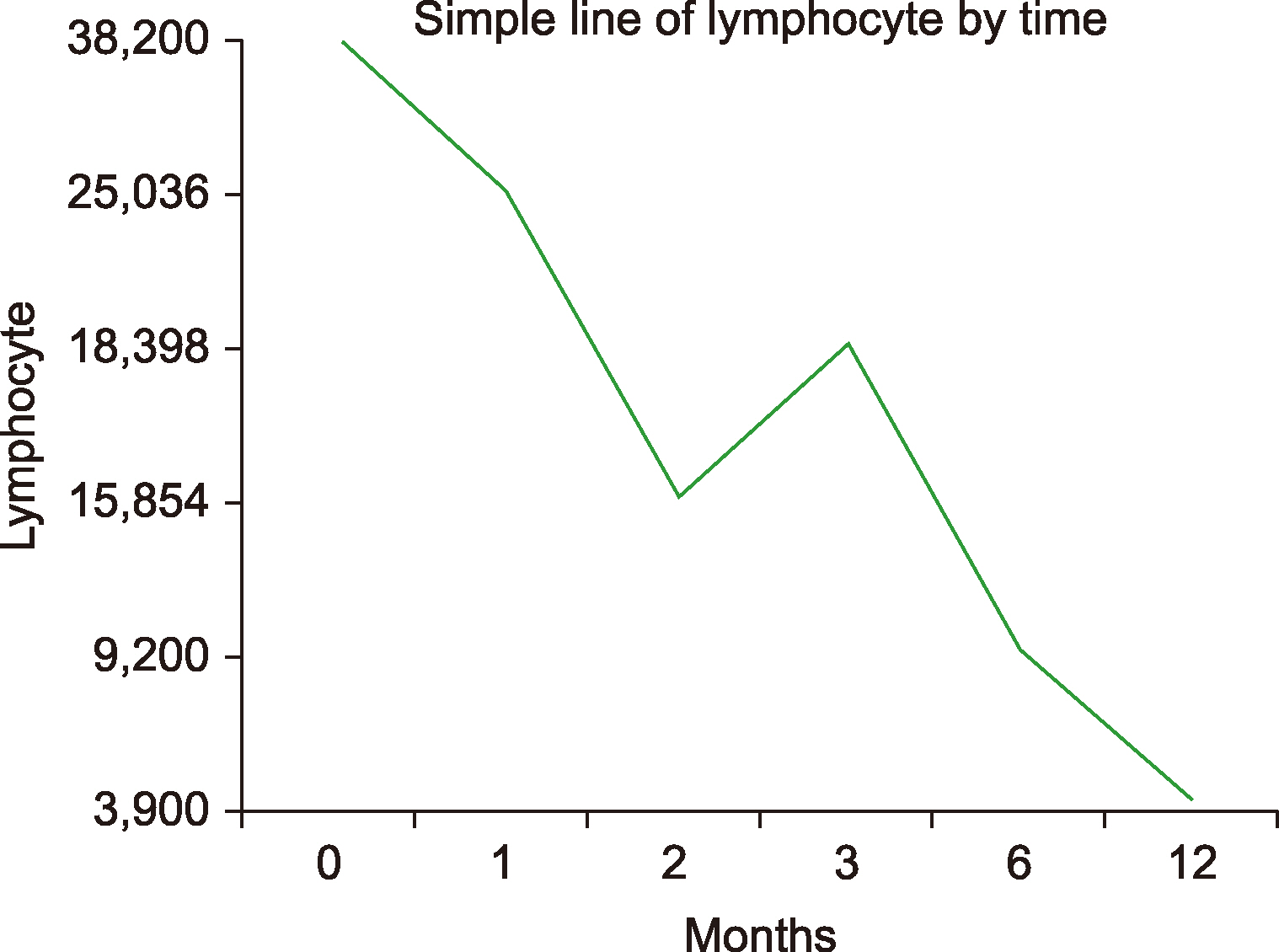
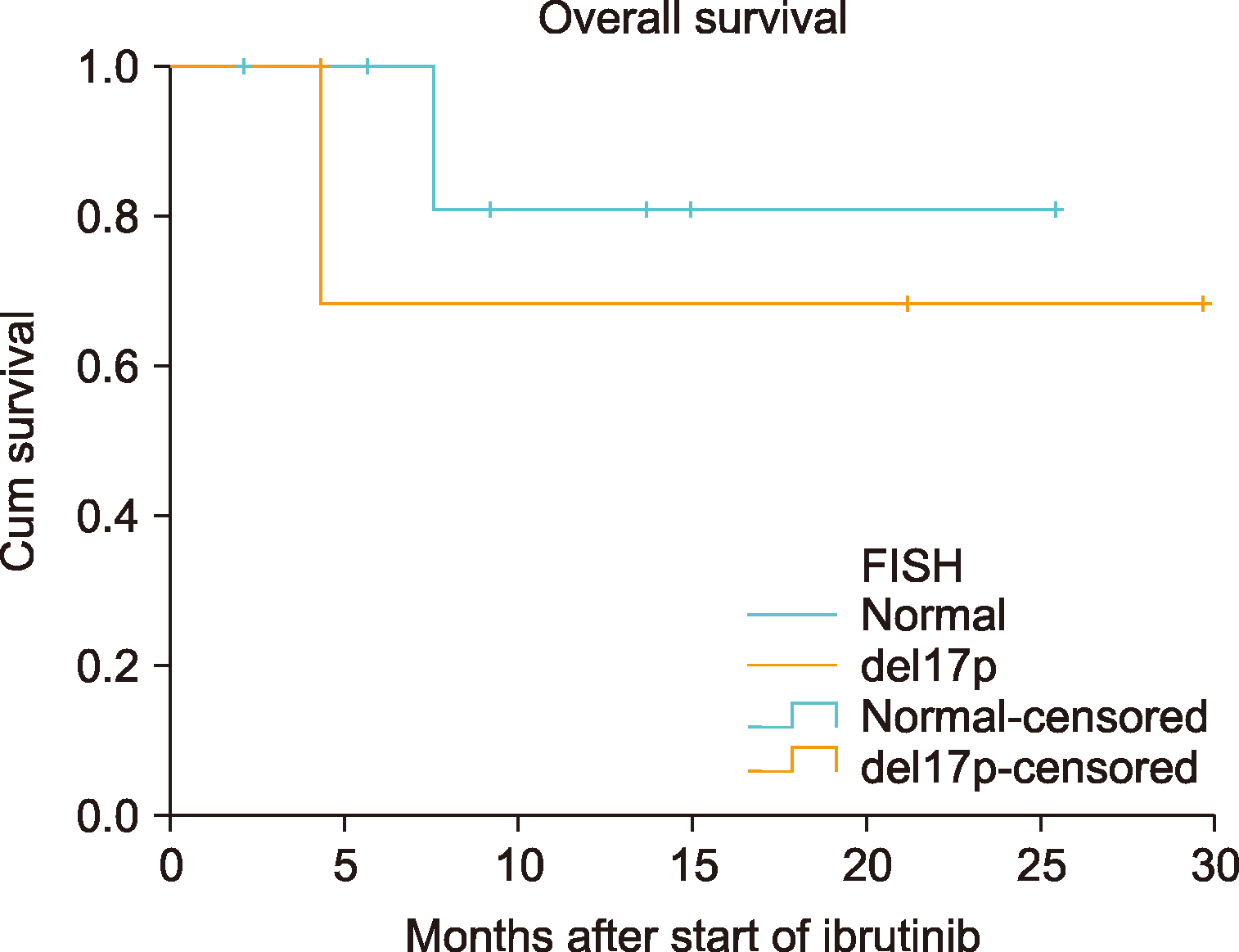
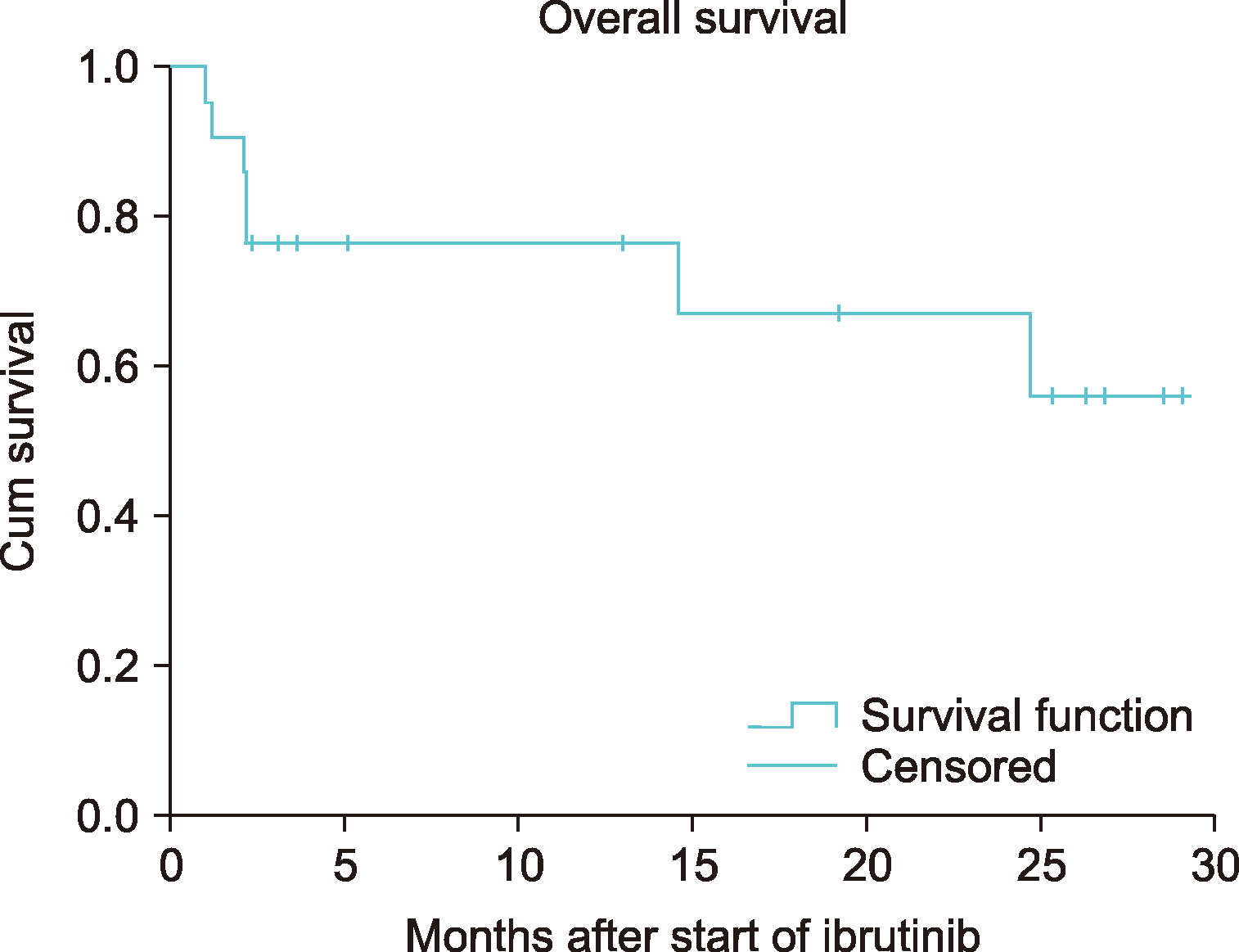
Of the 32 patients, 11 had CLL and 21 had other B-cell lymphomas. Patients with CLL were prescribed ibrutinib for a median of 4 months (range, 2‒18). In this group, diarrhea was observed in 3 (27.3%), pneumonia in 3 (27.3%), and thrombocytopenia and/or neutropenia in 2 (18.2%) patients. The overall response rate (ORR) was 85.6 % [28.5 % complete response (CR) and 57.1 % partial response (PR)] in the final response assessment during treatment with ibrutinib. Among other types of B-cell lymphoma, seven (33.4%) of the 21 patients were diagnosed with MCL, 5 (23.8%) with DLBCL, 4 (19.0%) with MZL, 3 (14.3%) with WM, and 2 (9.5%) with FL, upon follow-up. The median treatment duration was 4 months (range, 1‒28) in this group. The most common adverse event was diarrhea: 8 (38.1%) patients. The ORR was 66.6% (20.0% CR and 46.6% PR) in the final response assessment during the treatment.
Conclusion
Ibrutinib is a good treatment option for CLL and other B-cell lymphomas, with an acceptable side effect profile, and high and promising CR/PR response rates. Ibrutinib treatment at an early stage decreases the burden of cytotoxic therapy in fragile patients, thereby, increasing their quality of life.
Figure
Cited by 1 articles
-
Tp53 disruptions: is there a marker of poor prognosis in chronic lymphoproliferative disorders?
Emanuele Cencini, Alberto Fabbri, Donatella Raspadori, Alessandro Gozzetti, Monica Bocchia
Blood Res. 2021;56(4):333-334. doi: 10.5045/br.2021.2020322.
Reference
-
1. Melchers F. 2015; Checkpoints that control B cell development. J Clin Invest. 125:2203–10. DOI: 10.1172/JCI78083. PMID: 25938781. PMCID: PMC4497745.
Article2. Khan WN. 2001; Regulation of B lymphocyte development and activation by Bruton's tyrosine kinase. Immunol Res. 23:147–56. DOI: 10.1385/IR:23:2-3:147. PMID: 11444380.
Article3. Wiestner A. 2013; Targeting B-cell receptor signaling for anticancer therapy: the Bruton's tyrosine kinase inhibitor ibrutinib induces impressive responses in B-cell malignancies. J Clin Oncol. 31:128–30. DOI: 10.1200/JCO.2012.44.4281. PMID: 23045586.
Article4. Shankland KR, Armitage JO, Hancock BW. 2012; Non-Hodgkin lymphoma. Lancet. 380:848–57. DOI: 10.1016/S0140-6736(12)60605-9. PMID: 28153383.
Article5. Pan Z, Scheerens H, Li SJ, et al. 2007; Discovery of selective irreversible inhibitors for Bruton's tyrosine kinase. ChemMedChem. 2:58–61. DOI: 10.1002/cmdc.200600221. PMID: 17154430.
Article6. Byrd JC, Brown JR, O'Brien S, et al. 2014; Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 371:213–23. DOI: 10.1056/NEJMoa1400376. PMID: 24881631. PMCID: PMC4134521.7. Wang ML, Blum KA, Martin P, et al. 2015; Long-term follow-up of MCL patients treated with single-agent ibrutinib: updated safety and efficacy results. Blood. 126:739–45. DOI: 10.1182/blood-2015-03-635326. PMID: 26059948. PMCID: PMC4528064.
Article8. Fowler NH, Advani RH, Sharman J, et al. 2012; The Bruton's tyrosine kinase inhibitor ibrutinib (PCI-32765) is active and tolerated in relapsed follicular lymphoma. Blood (ASH Annual Meeting Abstracts). 120(Suppl):156. DOI: 10.1182/blood.V120.21.156.156.
Article9. Treon SP, Tripsas CK, Meid K, et al. 2015; Ibrutinib in previously treated Waldenström's macroglobulinemia. N Engl J Med. 372:1430–40. DOI: 10.1056/NEJMoa1501548. PMID: 25853747.10. Denlinger NM, Epperla N, William BM. 2018; Management of relapsed/refractory marginal zone lymphoma: focus on ibrutinib. Cancer Manag Res. 10:615–24. DOI: 10.2147/CMAR.S133291. PMID: 29628774. PMCID: PMC5877869.
Article11. Wilson WH, Young RM, Schmitz R, et al. 2015; Targeting B cell receptor signaling with ibrutinib in diffuse large B cell lymphoma. Nat Med. 21:922–6. DOI: 10.1038/nm.3884. PMID: 26193343.12. Singh J, Petter RC, Kluge AF. 2010; Targeted covalent drugs of the kinase family. Curr Opin Chem Biol. 14:475–80. DOI: 10.1016/j.cbpa.2010.06.168. PMID: 20609616.
Article13. Schmidt U, Boucheron N, Unger B, Ellmeier W. 2004; The role of Tec family kinases in myeloid cells. Int Arch Allergy Immunol. 134:65–78. DOI: 10.1159/000078339. PMID: 15133303.
Article14. Grabinski N, Ewald F. 2014; Ibrutinib (ImbruvicaTM) potently inhibits ErbB receptor phosphorylation and cell viability of ErbB2- positive breast cancer cells. Invest New Drugs. 32:1096–104. DOI: 10.1007/s10637-014-0141-2. PMID: 25081321.15. McMullen JR, Boey EJ, Ooi JY, Seymour JF, Keating MJ, Tam CS. 2014; Ibrutinib increases the risk of atrial fibrillation, potentially through inhibition of cardiac PI3K-Akt signaling. Blood. 124:3829–30. DOI: 10.1182/blood-2014-10-604272. PMID: 25498454.
Article16. Byrd JC, Furman RR, Coutre SE, et al. 2013; Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 369:32–42. DOI: 10.1056/NEJMoa1215637. PMID: 23782158. PMCID: PMC3772525.
Article17. Mato AR, Nabhan C, Thompson MC, et al. 2018; Toxicities and outcomes of 616 ibrutinib-treated patients in the United States: a real-world analysis. Haematologica. 103:874–9. DOI: 10.3324/haematol.2017.182907. PMID: 29419429. PMCID: PMC5927982.
Article18. Hallek M, Cheson BD, Catovsky D, et al. 2018; iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood. 131:2745–60. DOI: 10.1182/blood-2017-09-806398. PMID: 29540348.
Article19. Cheson BD, Ansell S, Schwartz L, et al. 2016; Refinement of the Lugano Classification lymphoma response criteria in the era of immunomodulatory therapy. Blood. 128:2489–96. DOI: 10.1182/blood-2016-05-718528. PMID: 27574190.
Article20. Smith A, Howell D, Patmore R, Jack A, Roman E. 2011; Incidence of haematological malignancy by sub-type: a report from the Haematological Malignancy Research Network. Br J Cancer. 105:1684–92. DOI: 10.1038/bjc.2011.450. PMID: 22045184. PMCID: PMC3242607.
Article21. Eichhorst B, Fink AM, Busch R, et al. 2013; Chemoimmunotherapy with fludarabine (F), cyclophosphamide (C), and rituximab (R) (FCR) versus bendamustine and rituximab (BR) in previously untreated and physically fit patients (pts) with advanced chronic lymphocytic leukemia (CLL): results of a planned interim analysis of the CLL10 Trial, an international, randomized study of the German CLL Study Group (GCLLSG). Blood (ASH Annual Meeting Abstracts). 122(Suppl):526. DOI: 10.1182/blood.V122.21.526.526.
Article22. Burger JA, Tedeschi A, Barr PM, et al. 2015; Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med. 373:2425–37. DOI: 10.1056/NEJMoa1509388. PMID: 26639149. PMCID: PMC4722809.
Article23. Brown JR, Hillmen P, O'Brien S, et al. 2018; Extended follow-up and impact of high-risk prognostic factors from the phase 3 RESONATE study in patients with previously treated CLL/SLL. Leukemia. 32:83–91. DOI: 10.1038/leu.2017.175. PMID: 28592889. PMCID: PMC5770586.
Article24. Winqvist M, Asklid A, Andersson PO, et al. 2016; Real-world results of ibrutinib in patients with relapsed or refractory chronic lymphocytic leukemia: data from 95 consecutive patients treated in a compassionate use program. A study from the Swedish Chronic Lymphocytic Leukemia Group. Haematologica. 101:1573–80. DOI: 10.3324/haematol.2016.144576. PMID: 27198718. PMCID: PMC5479603.25. Burger JA, Barr PM, Robak T, et al. 2020; Long-term efficacy and safety of first-line ibrutinib treatment for patients with CLL/SLL: 5 years of follow-up from the phase 3 RESONATE-2 study. Leukemia. 34:787–98. DOI: 10.1038/s41375-019-0602-x. PMID: 31628428. PMCID: PMC7214263.
Article26. Bartlett NL, LaPlant BR, Qi J, et al. 2014; Ibrutinib monotherapy in relapsed/refractory follicular lymphoma (FL): preliminary results of a phase 2 consortium (P2C) trial. Blood (ASH Annual Meeting Abstracts). 124(Suppl):800. DOI: 10.1182/blood.V124.21.800.800.
Article27. Jeon YW, Yoon S, Min GJ, et al. 2019; Clinical outcomes for ibrutinib in relapsed or refractory mantle cell lymphoma in real-world experience. Cancer Med. 8:6860–70. DOI: 10.1002/cam4.2565. PMID: 31560165. PMCID: PMC6853811.
Article28. Noy A, de Vos S, Thieblemont C, et al. 2017; Targeting Bruton tyrosine kinase with ibrutinib in relapsed/refractory marginal zone lymphoma. Blood. 129:2224–32. DOI: 10.1182/blood-2016-10-747345. PMID: 28167659. PMCID: PMC5399483.
Article29. Tillman BF, Pauff JM, Satyanarayana G, Talbott M, Warner JL. 2018; Systematic review of infectious events with the Bruton tyrosine kinase inhibitor ibrutinib in the treatment of hematologic malignancies. Eur J Haematol. 100:325–34. DOI: 10.1111/ejh.13020. PMID: 29285806.
Article30. Wang ML, Rule S, Martin P, et al. 2013; Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 369:507–16. DOI: 10.1056/NEJMoa1306220. PMID: 23782157. PMCID: PMC4513941.31. Bartlett NL, Costello BA, LaPlant BR, et al. 2018; Single-agent ibrutinib in relapsed or refractory follicular lymphoma: a phase 2 consortium trial. Blood. 131:182–90. DOI: 10.1182/blood-2017-09-804641. PMID: 29074501. PMCID: PMC5757691.
Article32. Dimopoulos MA, Trotman J, Tedeschi A, et al. 2017; Ibrutinib for patients with rituximab-refractory Waldenström's macroglobulinaemia (iNNOVATE): an open-label substudy of an international, multicentre, phase 3 trial. Lancet Oncol. 18:241–50. DOI: 10.1016/S1470-2045(16)30632-5. PMID: 27956157.
Article33. Fowler N, Nastoupil L, de Vos S, et al. 2015; Ibrutinib plus rituximab in treatment-naive patients with follicular lymphoma: results from a multicenter, phase 2 study. Blood (ASH Annual Meeting Abstracts). 126(Suppl):470. DOI: 10.1182/blood.V126.23.470.470.
Article34. Wadhwa PD, Morrison VA. 2006; Infectious complications of chronic lymphocytic leukemia. Semin Oncol. 33:240–9. DOI: 10.1053/j.seminoncol.2005.12.013. PMID: 16616071.
Article35. Morrison VA. 2009; Infectious complications in patients with chronic lymphocytic leukemia: pathogenesis, spectrum of infection, and approaches to prophylaxis. Clin Lymphoma Myeloma. 9:365–70. DOI: 10.3816/CLM.2009.n.071. PMID: 19858055.
Article36. Molica S. 1994; Infections in chronic lymphocytic leukemia: risk factors, and impact on survival, and treatment. Leuk Lymphoma. 13:203–14. DOI: 10.3109/10428199409056283. PMID: 8049645.
Article37. UK CLL Forum. 2016; Ibrutinib for relapsed/refractory chronic lymphocytic leukemia: a UK and Ireland analysis of outcomes in 315 patients. Haematologica. 101:1563–72. DOI: 10.3324/haematol.2016.147900. PMID: 27756834. PMCID: PMC5479600.38. Long M, Beckwith K, Do P, et al. 2017; Ibrutinib treatment improves T cell number and function in CLL patients. J Clin Invest. 127:3052–64. DOI: 10.1172/JCI89756. PMID: 28714866. PMCID: PMC5531425.
Article39. Wiczer TE, Levine LB, Brumbaugh J, et al. 2017; Cumulative incidence, risk factors, and management of atrial fibrillation in patients receiving ibrutinib. Blood Adv. 1:1739–48. DOI: 10.1182/bloodadvances.2017009720. PMID: 29296820. PMCID: PMC5728342.
Article40. Friedberg JW, Sharman J, Sweetenham J, et al. 2010; Inhibition of Syk with fostamatinib disodium has significant clinical activity in non-Hodgkin lymphoma and chronic lymphocytic leukemia. Blood. 115:2578–85. DOI: 10.1182/blood-2009-08-236471. PMID: 19965662. PMCID: PMC2852362.
Article41. Brown JR, Byrd JC, Coutre SE, et al. 2014; Idelalisib, an inhibitor of phosphatidylinositol 3-kinase p110d, for relapsed/refractory chronic lymphocytic leukemia. Blood. 123:3390–7. DOI: 10.1182/blood-2013-11-535047. PMID: 24615777. PMCID: PMC4123414.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Efficacy and Safety of Dupilumab for the Treatment of Moderate to Severe Atopic Dermatitis in Korea: A Real-World Data in a Single Medical Center
- A retrospective analysis of ibrutinib outcomes in relapsed or refractory mantle cell lymphoma
- Novel Agents in the Treatment of Chronic Lymphocytic Leukemia
- Progressive Multifocal Leukoencephalopathy after Ibrutinib Therapy for Chronic Lymphocytic Leukemia
- Mantle cell lymphoma relapsed after autologous stem cell transplantation: a single-center experience